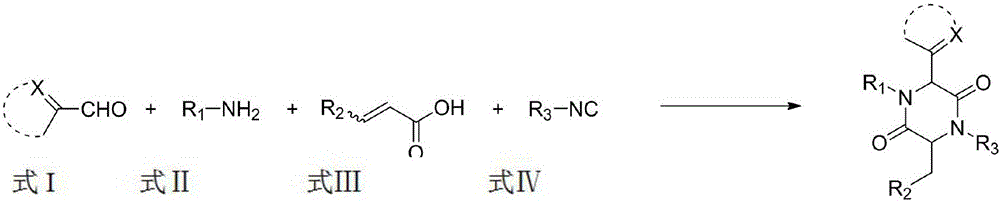
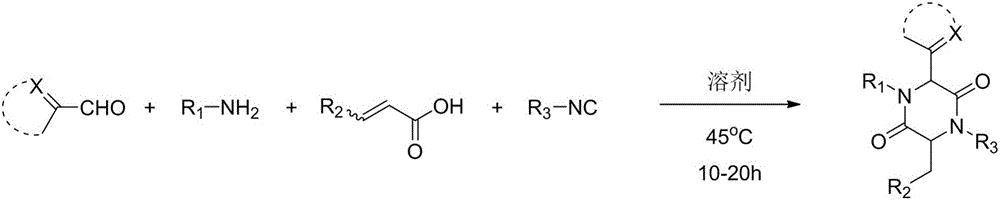
A method for synthesizing 2,5-dicarbonylpiperazines
A technology for dicarbonylpiperazine and compounds, applied in the field of organic synthesis, can solve the problems of harsh reaction conditions, failure to obtain products and the like, and achieve the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
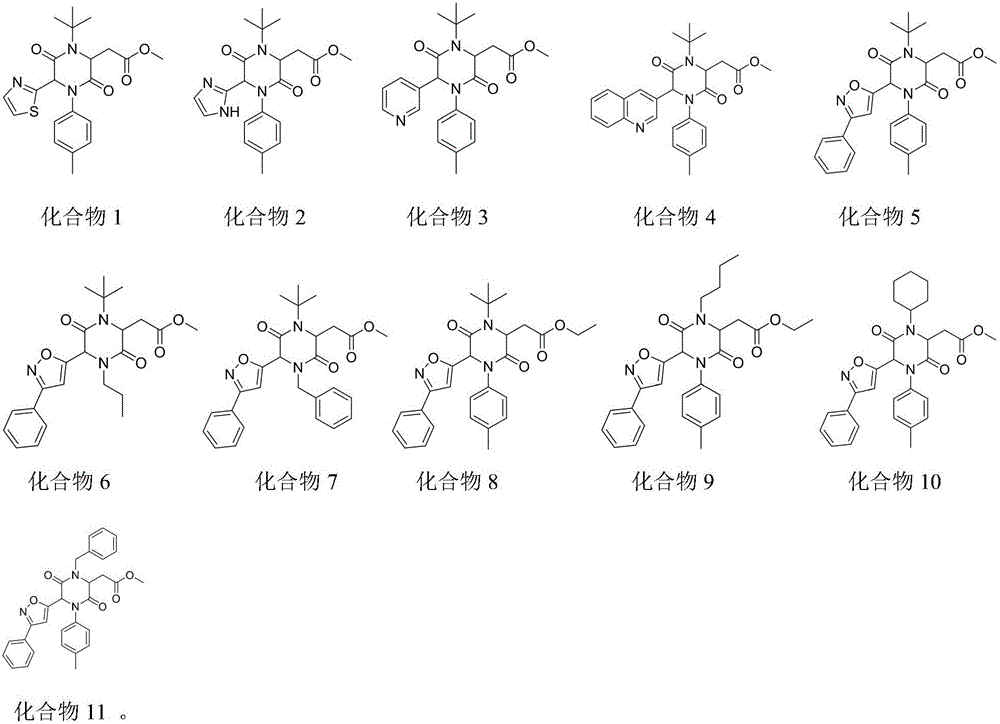
Examples
Embodiment 1
[0029] Add 2.0mL of methanol to a round bottom flask, and add in sequence, 22.6mg (0.20mmol) of thiazole-2-aldehyde, 21.4mg (0.20mmol) of p-toluidine, 26.0mg (0.20mmol) of monomethyl maleate and tert-butyl iso Nitrile 16.6mg (0.24mmol), and then heated to 45 ° C reaction, 10-20h after the point plate detection reaction is complete, with ethyl acetate: dichloromethane: petroleum ether = 1:1:10 to 1:1:5 as Mobile phase, column chromatography yielded 172.2 mg of compound, yield: 87%.
[0030] Synthesis of compound 1 (structure shown in the figure below).
[0031]
[0032] The synthetic method of embodiment 1 is the same as above-mentioned synthetic general method.
[0033] White solid; Yield: 87%.
[0034] 1 H NMR (400MHz, DMSO-d 6 ):δ8.87(s,0.5H,H a ),8.59(s,0.5H,H a ), 7.90(t, J=3.2Hz, 1H, thiazole), 7.83(t, J=3.6Hz, 1H, thiazole), 7.21~6.92(m, 4H, aryl), 4.12(dd, J=5.2, 10.8 Hz,0.5H,H b ),3.87(dd,J=6.8,8.8Hz,0.5H,H b ),3.60(s,1.5H,-OC H 3 ),3.51(s,1.5H,-OC H 3 ...
Embodiment 2
[0036] Add 2.0mL methanol to the round bottom flask, add in sequence, imidazole-2-carboxaldehyde 19.2mg (0.20mmol), p-toluidine 21.4mg (0.20mmol), monomethyl maleate 26.0mg (0.20mmol) and tert-butyl iso Nitrile 16.6mg (0.24mmol), and then heated to 45 ° C reaction, 10-20h after the point plate detection reaction is complete, with ethyl acetate: dichloromethane: petroleum ether = 1:1:10 to 1:1:5 as Mobile phase, column chromatography yielded 253.4 mg of compound, yield: 67%.
[0037] Synthesis of compound 2 (structure shown in the figure below).
[0038]
[0039] The synthetic method of embodiment 2 is the same as above-mentioned synthetic general method.
[0040] White solid; Yield: 67%.
[0041] 1 H NMR (400MHz, DMSO-d 6 ):δ12.64(s,0.3H,N H ),12.51(s,0.7H,N H ),10.50(s,0.3H,H a ),10.37(s,0.7H,H a ),7.21~6.92(m,6H,imidazol and aryl overlap),3.87~3.79(m,1H,H b ),3.55(s,1H,-OC H 3 ),3.47(s,2H,-OC H 3 ),2.87~2.69(m,1.3H,H c ),2.21(s,1H,-C H 3 ),2.20(s,2H,-C H ...
Embodiment 3
[0043] Add 2.0mmL of methanol to the round bottom flask, add successively, pyridine-2-carbaldehyde 21.4mg (0.20mmol), p-toluidine 21.4mg (0.20mmol), monomethyl maleate 26.0mg (0.20mmol) and tert-butyl iso Nitrile 16.6mg (0.24mmol), and then heated to 45 ° C reaction, 10-20h after the point plate detection reaction is complete, with ethyl acetate: dichloromethane: petroleum ether = 1:1:10 to 1:1:5 as Mobile phase, column chromatography obtained 350.7 mg of compound, yield: 62%.
[0044] Synthesis of compound 3 (structure shown in the figure below).
[0045]
[0046] The synthetic method of embodiment 3 is the same as above-mentioned synthetic general method.
[0047] White solid; Yield: 62%.
[0048] 1 H NMR (400MHz, DMSO-d 6 ):δ9.33(s,0.2H,H a ),9.08(s,0.8H,H a ), 8.47(t, J=4.4Hz, 1H, Py), 7.91~7.86(m, 1H, Py), 7.50~7.42(m, 1H, Py), 7.34(d, J=8.8Hz, 0.8H, Py),7.13~7.05(m,4H,aryl),4.00(dd,J=5.2,10.8Hz,0.8H,H b ),3.89(t,J=7.6Hz,0.2H,H b ),3.61(s,0.6H,-OC H 3 ),3.49(s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com