C-aryl glucoside SGLT2 (Sodium-Glucose Co-transporter 2) inhibitor
A glucopyranose, C2-C5 technology, applied in the preparation of antidiabetic drugs, in the field of type 2 sodium-glucose transporter inhibitors, can solve problems such as liver toxicity, hypoglycemia, and weight gain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
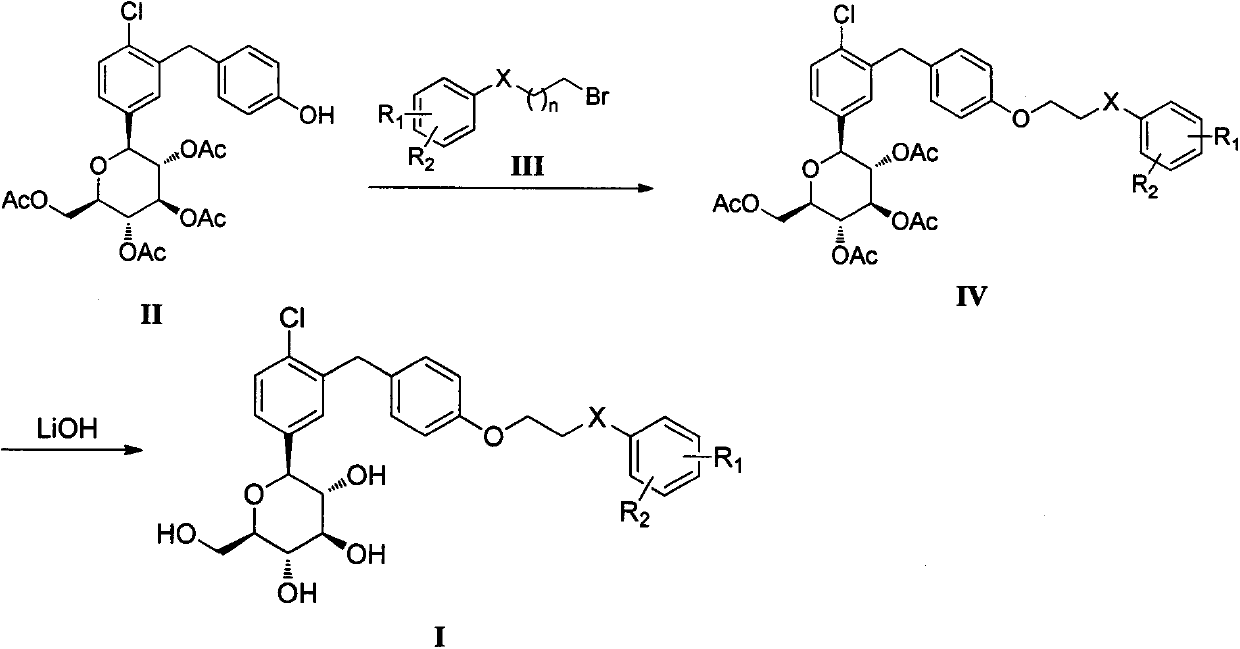
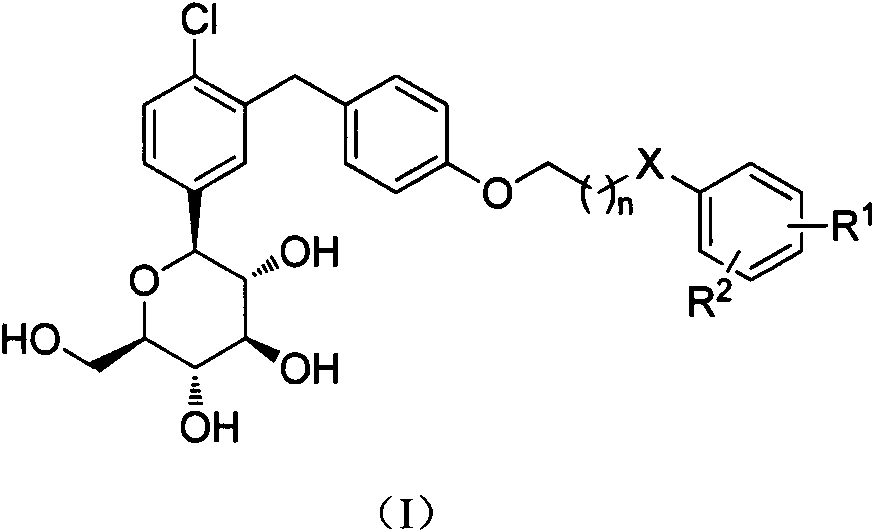
[0084] 1-{3-[4-(2-Phenoxy)ethoxy]benzyl-4-chloro}phenyl-1-deoxy-β-D-glucopyranose (I-1)
[0085] Compound II0.1g (0.18mmol) was dissolved in DMF, followed by adding K 2 CO 3 0.05g (0.36mmol), catalytic amount of TBAB and compound III-10.072g (0.36mmol), were reacted overnight at 80°C. After the reaction, add water to dissolve potassium carbonate, extract with ethyl acetate (20mL×3), combine organic phases, wash with 1N NaOH×2, 1N HCl×2, saturated NaCl×2, anhydrous NaCl 2 SO 4 dry. Suction filtration and column chromatography separation (petroleum ether: ethyl acetate = 2:1) gave white solid IV-180 mg, yield: 66.7%.
[0086] Compound IV-180mg (0.12mmol) was dissolved in 10mL tetrahydrofuran, methanol and water (2:3:1) three-component solvent, 0.006g (0.144mmol) LiOH hydrate was added, and reacted overnight at room temperature. After the reaction was completed, it was concentrated, dissolved in ethyl acetate, and dissolved in 5% KHSO 4 × 1, washed with saturated NaCl × 1, ...
Embodiment 2
[0091] 1-{3-{4-[2-(4-Methyl)phenoxy]ethoxy}benzyl-4-chloro}phenyl-1-deoxy-β-D-glucopyranose (I- 2)
[0092] Compound II0.26g (0.47mmol) was dissolved in DMF, followed by adding K 2 CO 3 0.13 g (0.95 mmol), catalytic amount of TBAB and 0.20 g (0.95 mmol) of compound III-2 were reacted overnight at 80°C. After the reaction, add water to dissolve potassium carbonate, extract with ethyl acetate (20mL×3), combine organic phases, wash with 1N NaOH×2, 1N HCl×2, saturated NaCl×2, anhydrous NaCl 2 SO 4 dry. Suction filtration and column chromatography separation (petroleum ether: ethyl acetate = 2:1) gave white solid IV-20.16g, yield: 50%.
[0093] Compound IV-20.16g (0.234mmol) was dissolved in 10mL tetrahydrofuran, methanol and water (2:3:1) three-component solvent, LiOH hydrate 0.012g (0.281mmol) was added, and reacted overnight at room temperature. After the reaction was completed, it was concentrated, dissolved in ethyl acetate, and dissolved in 5% KHSO 4 × 1, washed with s...
Embodiment 3
[0098] 1-{3-{4-[2-(2-Methyl)phenoxy]ethoxy}benzyl-4-chloro}phenyl-1-deoxy-β-D-glucopyranose (I- 3)
[0099] Compound II0.2g (0.36mmol) was dissolved in DMF, followed by adding K 2 CO 3 0.1 g (0.72 mmol), catalytic amount of TBAB and 0.16 g (0.0.72 mmol) of compound III-3 were reacted overnight at 80°C. After the reaction, add water to dissolve potassium carbonate, extract with ethyl acetate (20mL×3), combine the organic phases, wash with 1N NaOH×2, 1N HCl×2, saturated NaCl×2, anhydrous NaCl 2 SO 4 dry. Suction filtration, column chromatography separation (petroleum ether: ethyl acetate = 2:1), to obtain white solid IV-30.21g, yield: 84%.
[0100] Compound IV-30.2g (0.29mmol) was dissolved in 10mL tetrahydrofuran, methanol and water (2:3:1) three-component solvent, 0.015g (0.348mmol) LiOH hydrate was added, and reacted overnight at room temperature. After the reaction was completed, it was concentrated, dissolved in ethyl acetate, and dissolved in 5% KHSO 4 × 1, washed w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com