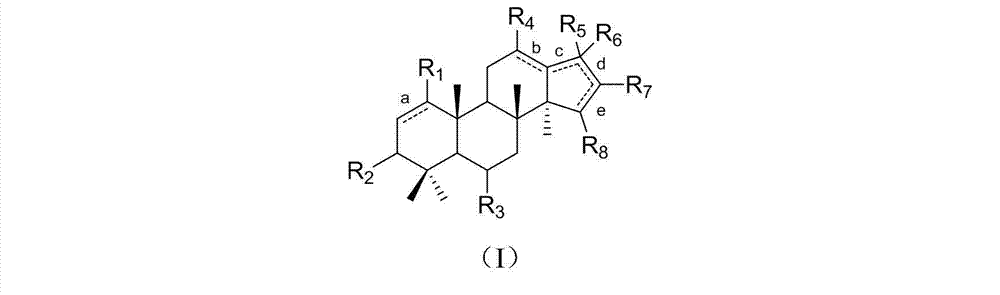
Dammarane triterpene derivative, pharmaceutical composition thereof, and applications of dammarane triterpene derivative in pharmacy
A technology for dammarane-type triterpenes and derivatives, which is applied in the field of dammarane triterpenoid derivatives and can solve problems such as no dammarane-type triterpenoids.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
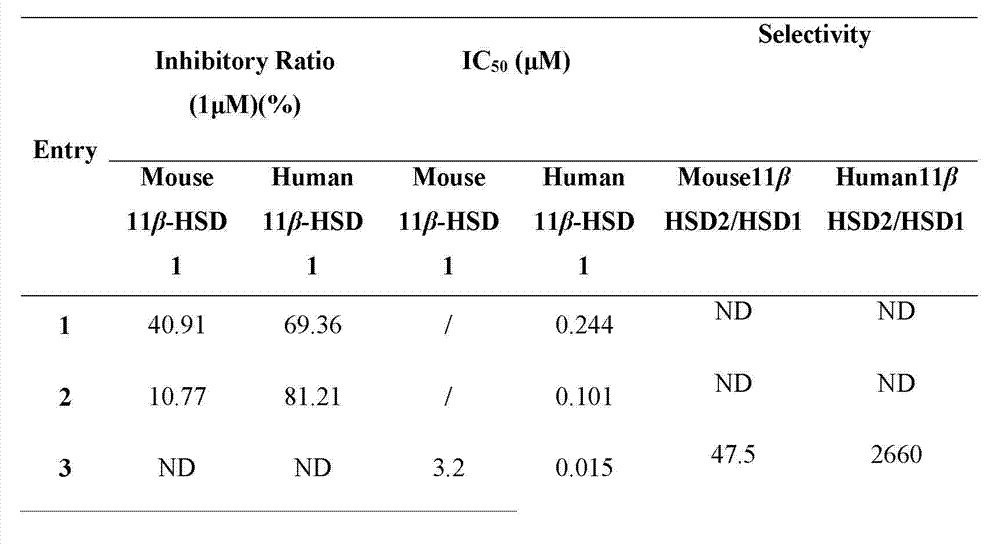
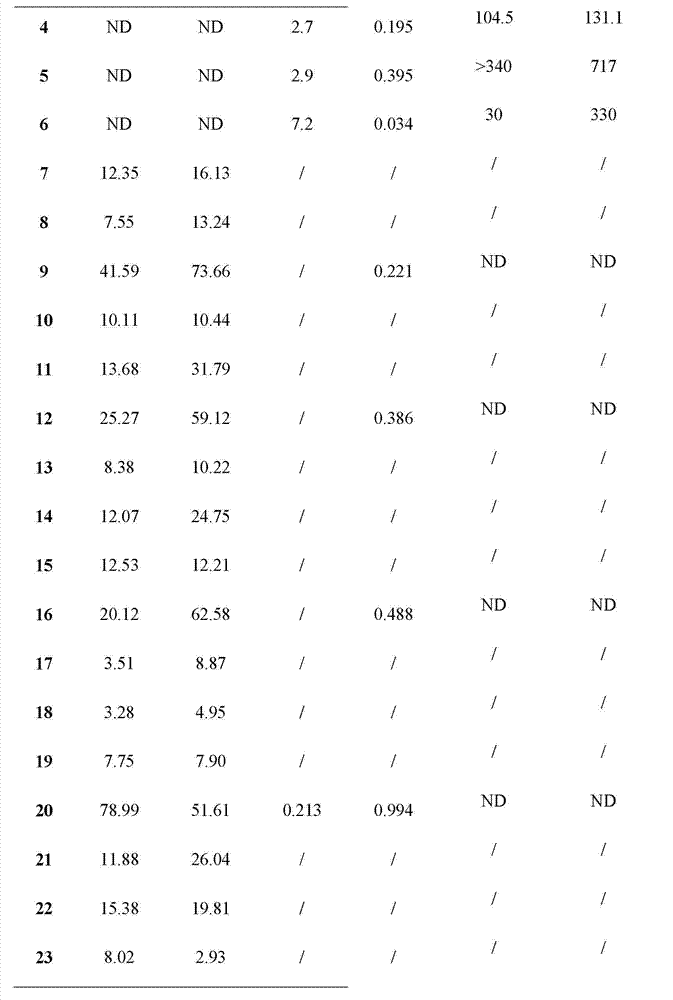
[0051] The compound dammarane-type triterpene derivatives of the present invention have obvious 11β-HSD1 inhibitory activity, and the experimental methods and results are as follows:
[0052] 1. Materials and methods:
[0053] 1. Sample and preparation:
[0054] The sample was colorless, and was dissolved in dimethyl sulfoxide (DMSO) to prepare a stock solution with a concentration of 10 mg / ml and stored in the dark for future use.
[0055] 2. Biological model 11β-HSD1:
[0056] Human 11β-HSD1; mouse 11β-HSD1.
[0057] 3. Experimental method:
[0058] (1) Using the SPA (Scintillation proximity assay, liquid scintillation proximity assay technology) method, all derivatives were screened for the inhibition of mouse 11β-HSD1 and human 11β-HSD1, and 1 μM was selected as the initial screening concentration. Derivatives with an inhibition rate of more than 50% in the initial screening were re-screened, and the dose-effect relationship study was carried out, and the IC50 value ...
Embodiment 2
[0068] Preparation of Compound 8:
[0069]
[0070]
[0071] Dissolve 20 g of 20(s)-protopanaxadiol (compound 7) in 450 ml of methanol, add an appropriate amount of Raney Ni, connect a hydrogen balloon, pump three times to remove the air in the system, stir at room temperature overnight, and filter with diatomaceous earth , the filtrate was collected, and the solvent was distilled off under reduced pressure to obtain compound 13 as a white solid. ESI: 485 [M+23] + , 1 H-NMR ( CDCl 3 , 500MHz) characteristic signal δ: 3.51-3.61 (1H, td, J=5.2 / 10.3 / 10.3, H-C(12)), 3.17-3.21 (1H, dd, J=4.9 / 11.2, H-C(3)), 1.99 -2.06 (1H, td, J=7.1 / 10.6 / 10.6, H-C(13)).
[0072] Dissolve 5.7 g of compound 13 in 120 ml of dichloromethane, add 10.27 ml of triethylamine at 0 o Stir for 10min at C, 0 o Slowly add 4.66ml Ac 2 80 ml of dichloromethane solution of O, stirred at room temperature for 4 h after dropping, washed 3 times with saturated NaCl solution, and washed the organic phase w...
Embodiment 3
[0074] Preparation of compounds 1 and 2:
[0075]
[0076]
[0077] Dissolve 15 g of compound 8 in 150 ml of pyridine, 0 o Slowly add 4 eq POCl dropwise at C 3 50 ml of pyridine solution, 40 o Stirred at C for 8h, diluted with ethyl acetate, and washed with saturated CuSO 4 The solution was washed 3 times, and the organic phase was washed with anhydrous NaSO 4 After drying, the solvent was distilled off under reduced pressure to obtain Compound 14 as a pale yellow solid. ESI: 551 [M+23] + , 1 H-NMR ( CDCl 3 , 500MHz) characteristic signal δ: 5.03-5.06 (1H, t, J=6.6, H-C(22)) 4.89-4.96 (1H, td, J=5.3 / 10.9 / 10.9, H-C(12)), 4.45-4.49 ( 1H, dd, J=4.7 / 11.2, H-C(3)), 2.44-2.57 (1H, m, H-C(17)).
[0078] Dissolve 4 g of compound 14 in 40 ml of CCl 4 , add 60 ml saturated NaHCO 3 solution with 8.09 g NaIO 4 , stirred for 15 minutes, added 0.198 g RuCl 3 ·nH 2 O in MeCN solution 40 ml, the above mixture was stirred at room temperature for 4 days in the dark, filtered...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com