Preparation method of acetylacetonatocarbonyltriphenylphosphine rhodium, and olefin hydroformylation method
A technology of acetone triphenylphosphine and acetone dicarbonyl rhodium is applied in a field of preparation and olefin hydroformylation, can solve the problems of low catalytic activity, decreased catalytic activity and the like, and achieves the effect of solving low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The invention provides a kind of preparation method of triphenylphosphine carbonyl rhodium acetylacetonate, it is characterized in that, the method comprises:
[0023] (1) Under the conditions of the precipitation reaction, the rhodium trichloride aqueous solution is contacted with the precipitating agent, so that the rhodium element is precipitated, and the precipitation of the rhodium-containing element is obtained;
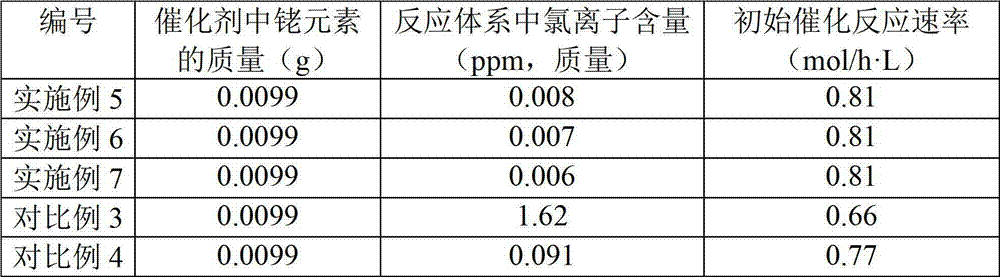
[0024](2) Washing the precipitate containing rhodium elements until the chloride ion content in the precipitate containing rhodium elements is lower than 1000ppm;
[0025] (3) Under the conditions of the reaction for synthesizing rhodium dicarbonyl acetylacetonate, dissolving the precipitation of the rhodium-containing element obtained in step (2) and then contacting acetylacetone to obtain a reaction solution containing rhodium dicarbonyl acetylacetonate;
[0026] (4) Under the conditions of the reaction for synthesizing rhodium acetylacetonate tripheny...
Embodiment 1
[0072] This example is used to illustrate the preparation method of triphenylphosphinecarbonylrhodium acetylacetonate provided by the present invention.
[0073] (1) Precipitation reaction: In a 1500ml beaker, add 250.00ml of rhodium trichloride aqueous solution with a Rh concentration of 1.000mol / L, under magnetic stirring, control the reaction temperature at 10°C, and drop NaOH with a concentration of 1.00mol / L About 750ml of solution, monitoring solution pH value change, stops dropwise when solution pH value=8.0, obtains the reaction solution that contains rhodium hydroxide precipitation.
[0074] (2) Washing the precipitate: filter the reaction solution containing the rhodium hydroxide precipitate under reduced pressure to separate the rhodium hydroxide precipitate. Wash three times with deionized water at a temperature of 10°C, and finally wash once with 10°C cyclohexane. After vacuum drying at 25°C, a yellow rhodium hydroxide precipitate was obtained. The content of ch...
Embodiment 2
[0082] This example is used to illustrate the preparation method of triphenylphosphinecarbonylrhodium acetylacetonate provided by the present invention.
[0083] (1) Precipitation reaction: In a 1000ml beaker, add 150.0ml of rhodium trichloride aqueous solution with a Rh concentration of 2.000mol / L. Under magnetic stirring, control the reaction temperature to be 7°C, add about 300ml of KOH solution with a concentration of 3.00mol / L dropwise, monitor the change in pH value of the solution, stop the dropwise addition when the pH value of the solution=7.0, and obtain a precipitate containing rhodium hydroxide reaction solution.
[0084] (2) Washing the precipitate: filter the reaction solution containing the rhodium hydroxide precipitate under reduced pressure to separate the rhodium hydroxide precipitate. Wash three times with deionized water at 8°C, and finally wash once with 7°C cyclohexane. After vacuum drying at 25°C, the resulting yellow rhodium hydroxide precipitates. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com