Benzimidazole derivatives, preparation method and medicinal use thereof
A technology of phenyl and compound, applied in the field of benzimidazole derivatives and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] (Isopropyloxycarbonyloxy)methyl-2-ethoxy-1-{[2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazole)- Synthesis of 3-yl)biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylate
[0088]
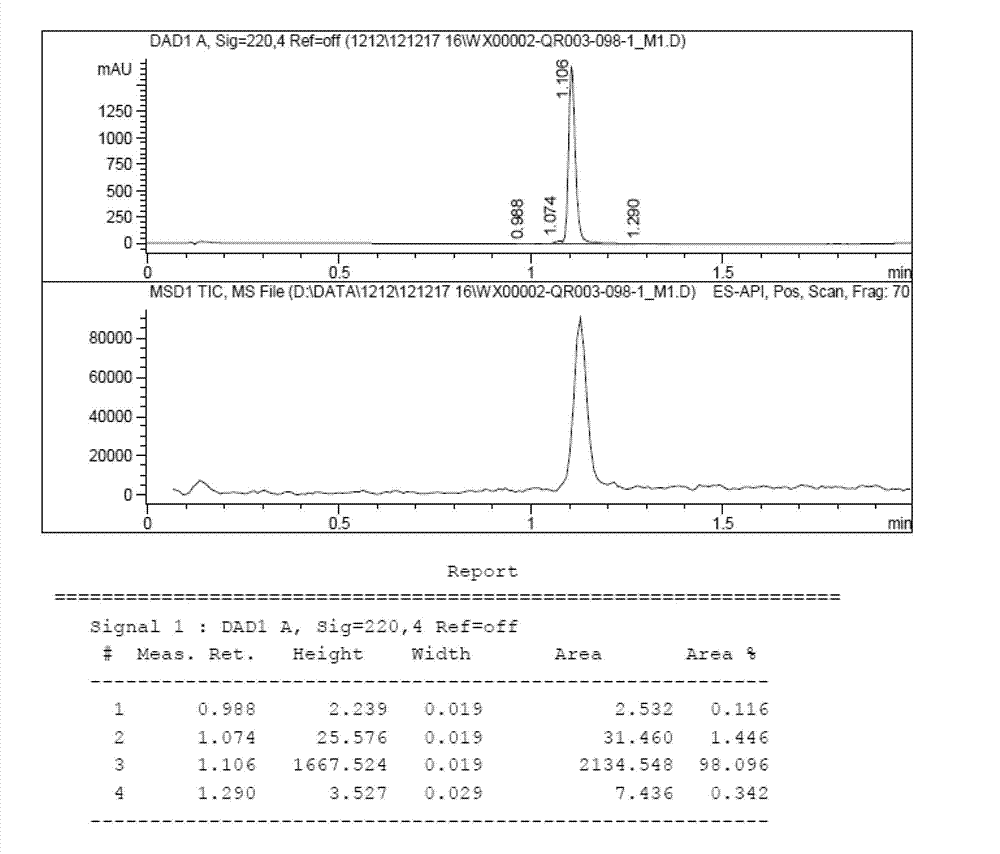
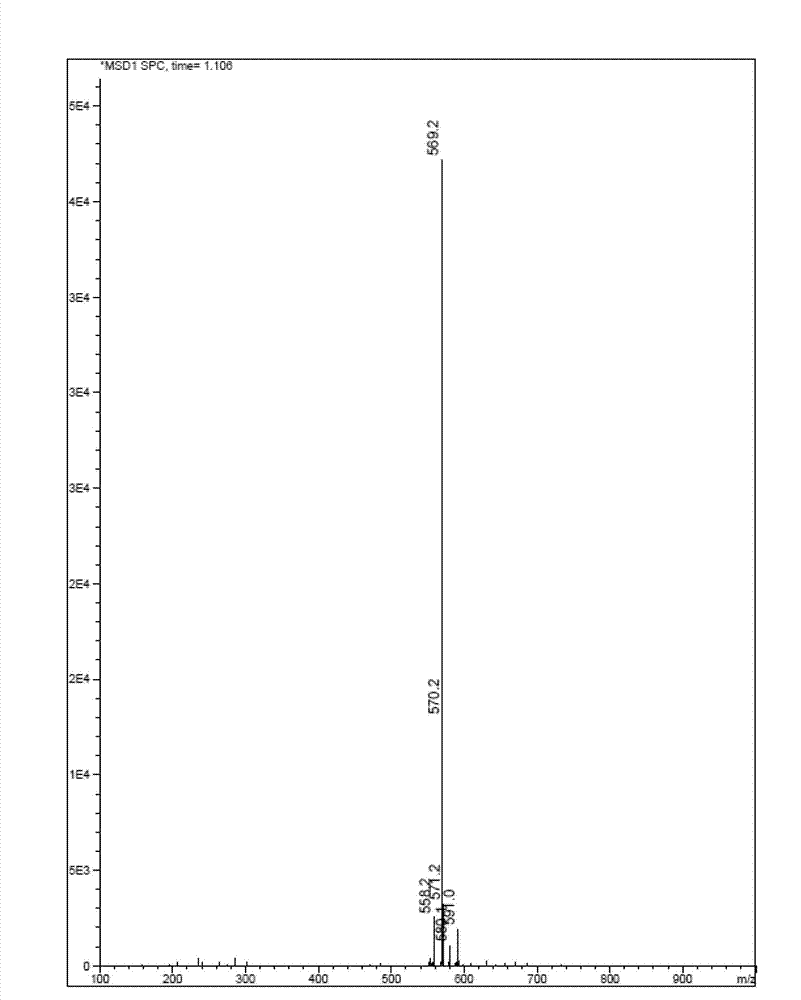
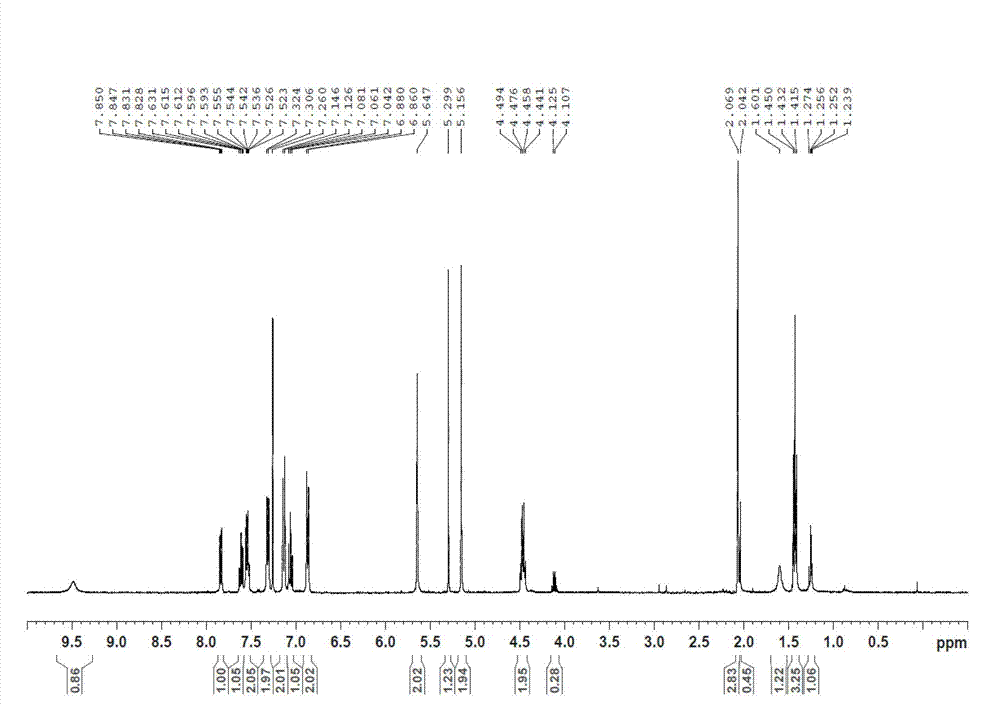
[0089] Dissolve QR01000-IN-01 (2.2 mmol, 1.0 equivalent) and QR01002-IN-01 (3.3 mmol, 1.5 equivalent) in 20 mL of N-methylpyrrolidone, add triethylamine (4.4 mmol, 2.0 equivalent), and heat to 65 °C, the reaction was monitored by TLC, and the reaction was complete. Water and ethyl acetate were added to the reaction liquid, extraction and separation were carried out, and the organic layer was washed with water and saturated brine. The organic layer was dried and concentrated, and purified by column chromatography to obtain the target compound QR01002, whose structure was double-confirmed by LCMS spectrum and H NMR spectrum.
Embodiment 2
[0091] 1-(isopropyloxycarbonyloxy)ethyl-2-ethoxy-1-{[2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazole Synthesis of )-3-yl)biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylate
[0092]
[0093] Dissolve QR01000-IN-01 (2.2 mmol, 1.0 equivalent) and QR01003-IN-01 (3.3 mmol, 1.5 equivalent) in 20 mL of N-methylpyrrolidone, add triethylamine (4.4 mmol, 2.0 equivalent), and heat to 65 °C, the reaction was monitored by TLC, and the reaction was complete. Water and ethyl acetate were added to the reaction liquid, extraction and separation were carried out, and the organic layer was washed with water and saturated brine. The organic layer was dried and concentrated, and purified by column chromatography to obtain the target compound QR01003, whose structure was double confirmed by LCMS spectrum and H NMR spectrum.
Embodiment 3
[0095] Acetoxyethyl-2-ethoxy-1-{[2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol)-3-yl)biphenyl- Synthesis of 4-yl]methyl}-1H-benzimidazole-7-carboxylate
[0096]
[0097] Dissolve QR01000-IN-01 (2.2mmol, 1.0eq) and QR01004-IN-03 (3.3mmol, 1.5eq) in 20mL of N-methylpyrrolidone, add triethylamine (4.4mmol, 2.0eq), heat to 65 °C, the reaction was monitored by TLC, and the reaction was complete. Water and ethyl acetate were added to the reaction liquid, extraction and separation were carried out, and the organic layer was washed with water and saturated brine. The organic layer was dried and concentrated, and purified by column chromatography to obtain the target compound QR01004, whose structure was double-confirmed by LCMS spectrum and H NMR spectrum.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com