Slow-release preparation of ornithine aspartate and preparation process thereof
A technology of ornithine aspartate and sustained-release preparations is applied in the field of ornithine-aspartate sustained-release capsule formulations, which can solve the problem that the controlled-release preparations of ornithine aspartate cannot be well realized, etc. problems, to achieve a wide range of social and economic benefits, easy to use, less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Preparation of drug-containing pellet core
[0047] Ornithine Aspartate 200g
[0048] Blank ball core 316g
[0049] 6% PVP solution (solvent is 85% ethanol) 190g
[0050] Preparation Process:
[0051] Pass ornithine aspartic acid through an 80-mesh sieve, weigh the prescribed amount, pour it into the lower hopper of the granulation coating machine, set the air inlet pressure and the rotating speed of the turntable, and then pour it into blank pellet cores for granulation. Spray 6% PVP solution (solvent is 85% ethanol), dry at 40°C, granulation is completed, and 500g of material is discharged.
Embodiment 2
[0053] (1) Preparation of drug-containing pellet core
[0054] Ornithine Aspartate 200g
[0055] Blank ball core 316g
[0056] 6% PVP solution (solvent is 85% ethanol) 180g
[0057] Preparation Process:
[0058] Pass ornithine aspartic acid through an 80-mesh sieve, weigh the prescribed amount, pour it into the lower hopper of the granulation coating machine, set the air inlet pressure and the rotating speed of the turntable, and then pour it into blank pellet cores for granulation. Spray 6% PVP solution (solvent is 85% ethanol), dry at 50°C, granulation is completed, and 495g of material is discharged.
Embodiment 3
[0060] (1) Preparation of drug-containing pellet core
[0061] Ornithine Aspartate 200g
[0062] Blank ball core 316g
[0063] 6% PVP solution (solvent is 85% ethanol) 200g
[0064] Preparation Process:
[0065] Put ornithine aspartic acid through 80 mesh sieve, weigh the prescription amount, pour it into the lower hopper of the granulation coating machine, set the air inlet pressure and the rotating speed of the turntable, then pour into the blank pellet core, spray 6% PVP solution ( The solvent is 85% ethanol), granulated. Dry at 45°C, the granulation is completed, and 508g is discharged.
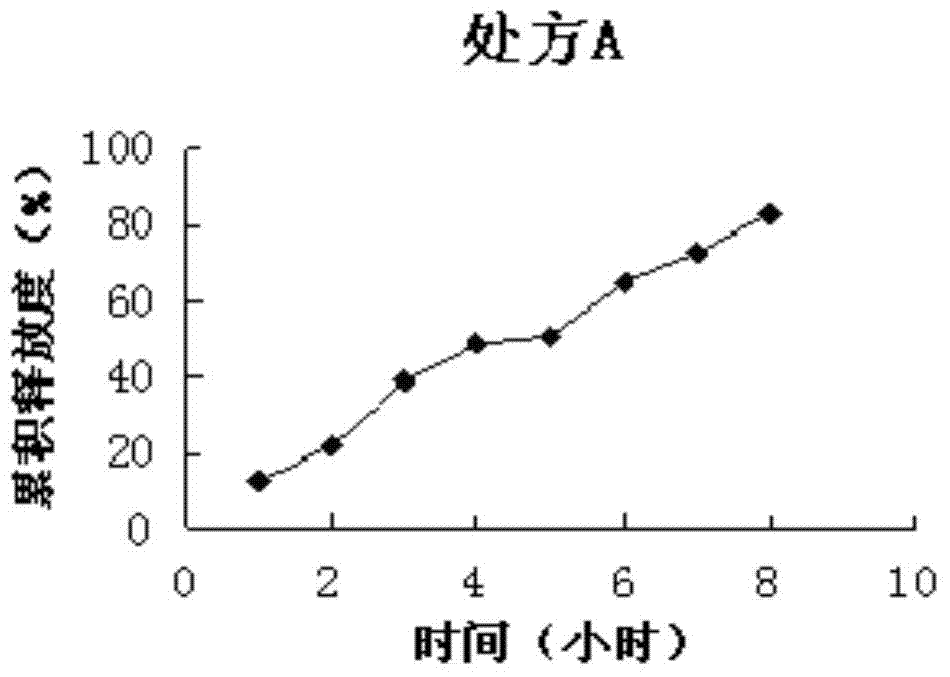
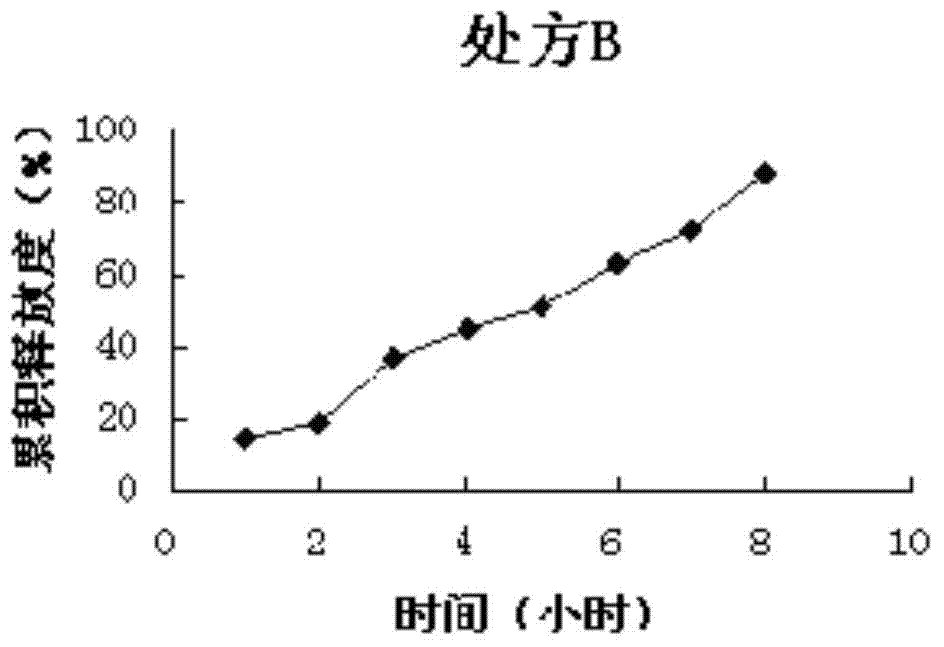
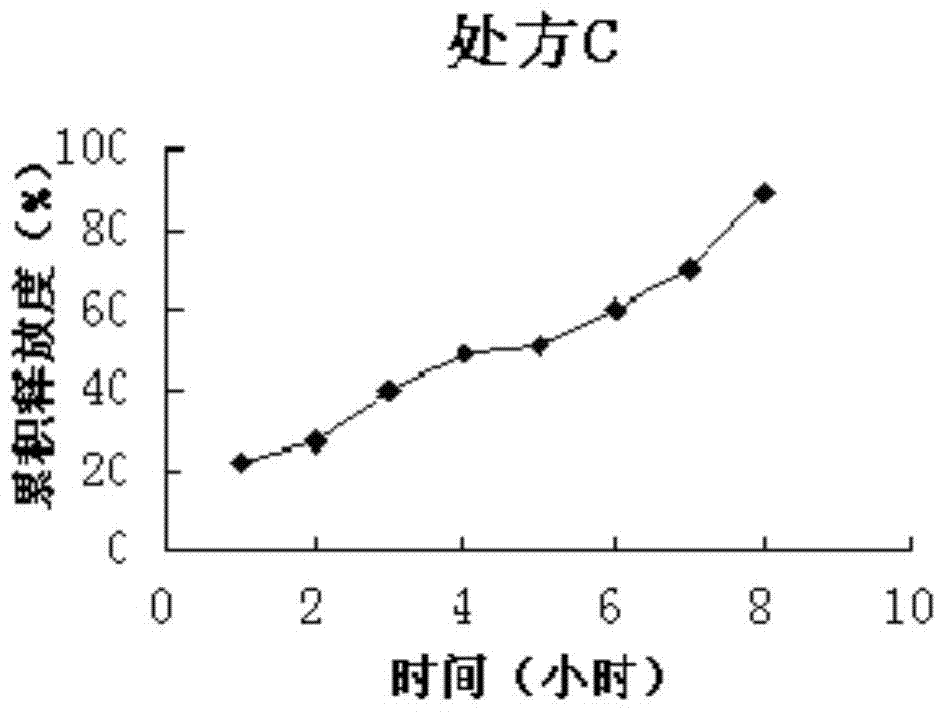
[0066] Sustained-release preparation formulation screening test (to determine the type and quantity of optimal sustained-release agent and plasticizer):
[0067] Prescription A:
[0068]
[0069] Preparation process: Weigh the pill core containing the prescription amount, pour it into the lower hopper of the granulation coating machine, set the air inlet pressure and the rotatin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com