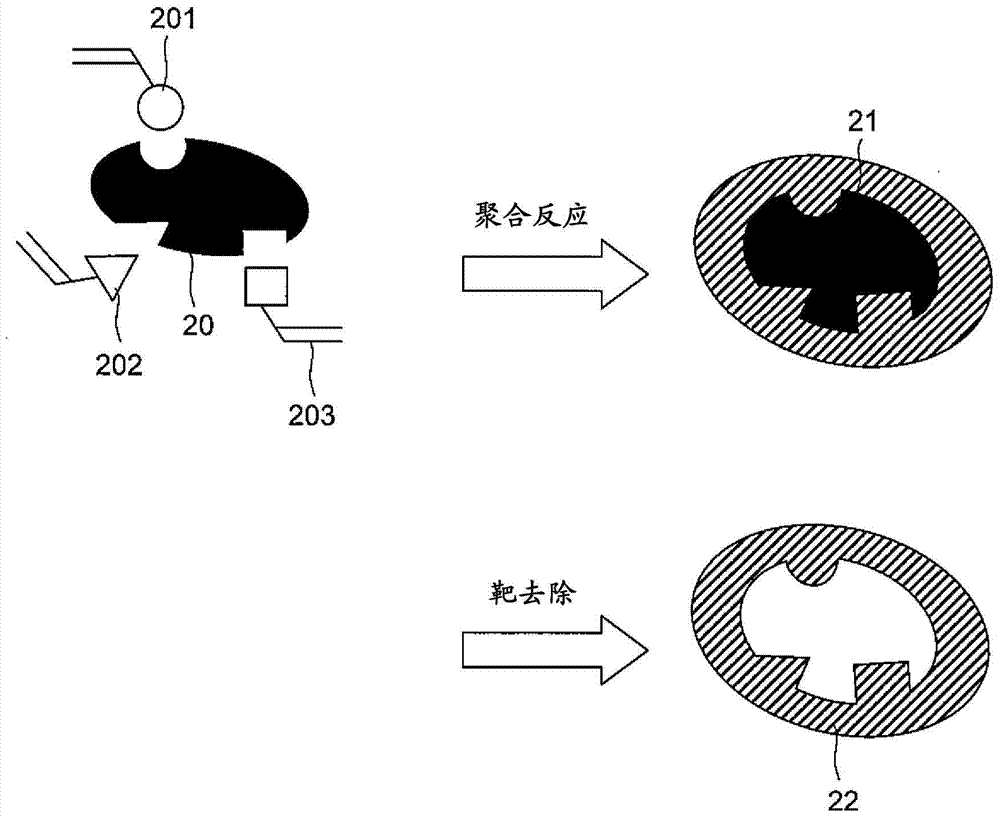
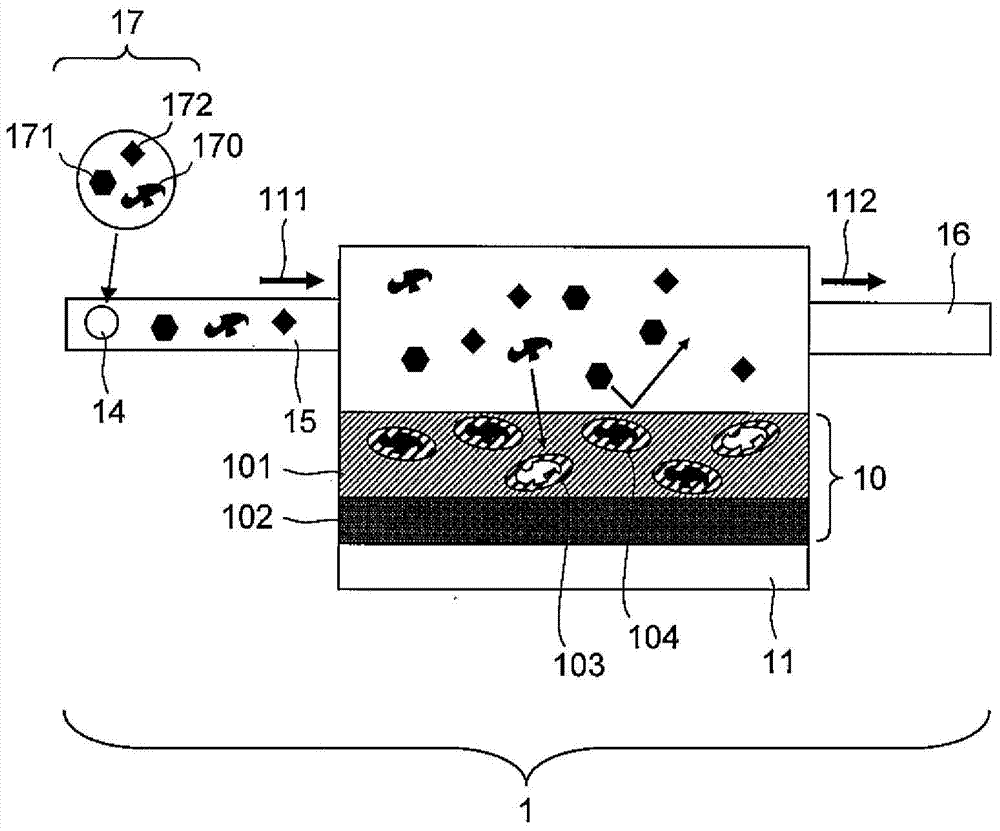
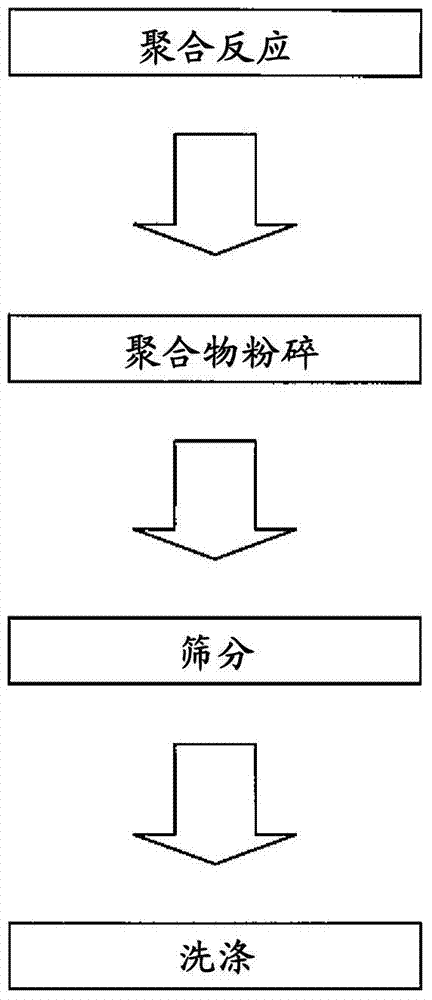
Molecular template and method for producing same
A technology of molecular template and manufacturing method, which is applied in the analysis of materials, material analysis by optical means, and analysis by chemical reaction of materials, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] The molecular template was synthesized by the method shown below using THF (tetrahydrofuran) as a solvent. First, to a mixture of cortisol: itaconic acid: styrene: divinylbenzene (DVB) = 1:4:4:20 (molar ratio), 2% V-70 (2,2'-azobis(4-methoxy-2,4-dimethylvaleronitrile)) as a polymerization initiator.
[0078] Specifically, the polymerization of molecular template polymer (MIP) was carried out according to the formulation in Table 1. Add 362.5mg (1mmol) of cortisol, 524mg (4mmol) of itaconic acid, 418mg (4mmol) of styrene, 2604mg (20mmol) of divinylbenzene (DVB) into a vial, dissolve in THF (20ml ), then move to In a test tube of 18 x 180 mm, 2,2'-azobis(4-methoxy-2,4-dimethylvaleronitrile) (V-70) (0.56 mmol) as a polymerization initiator was dissolved. A septum cap was capped and nitrogen substitution was performed, and polymerization was performed in a 30° C. water bath for 72 hours.
[0079]For a reference experiment, polymerization of a non-molecular template pol...
Embodiment 2
[0092] Methacrylation of Cortisol
[0093] In the above-mentioned Example 1, an example in which MIP was synthesized by utilizing hydrogen bonding between a molecular template raw material such as itaconic acid and cortisol as a template molecule was described. An example of MIP synthesis using a covalent bond between a raw material and a template molecule will be described below. The MIP produced in this Example 2 is called MIP1. First, when synthesizing MIP1, cortisol as a template molecule was converted in the following procedure.
[0094] First, under a nitrogen atmosphere, cortisol (2.5 mmol, 907 mg) was dissolved in dry THF (40 mL), triethylamine (30 mmol, 4.2 ml) was added and ice-cooled. Dry THF (40 mL) in which methacryloyl chloride (15 mmol, 1.5 ml) was dissolved was slowly added dropwise thereto, stirred at 0° C. for 1 hour, and then stirred at room temperature for 4 hours.
[0095] Next, ethyl acetate was added to the reaction liquid, and the organic phase was w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com