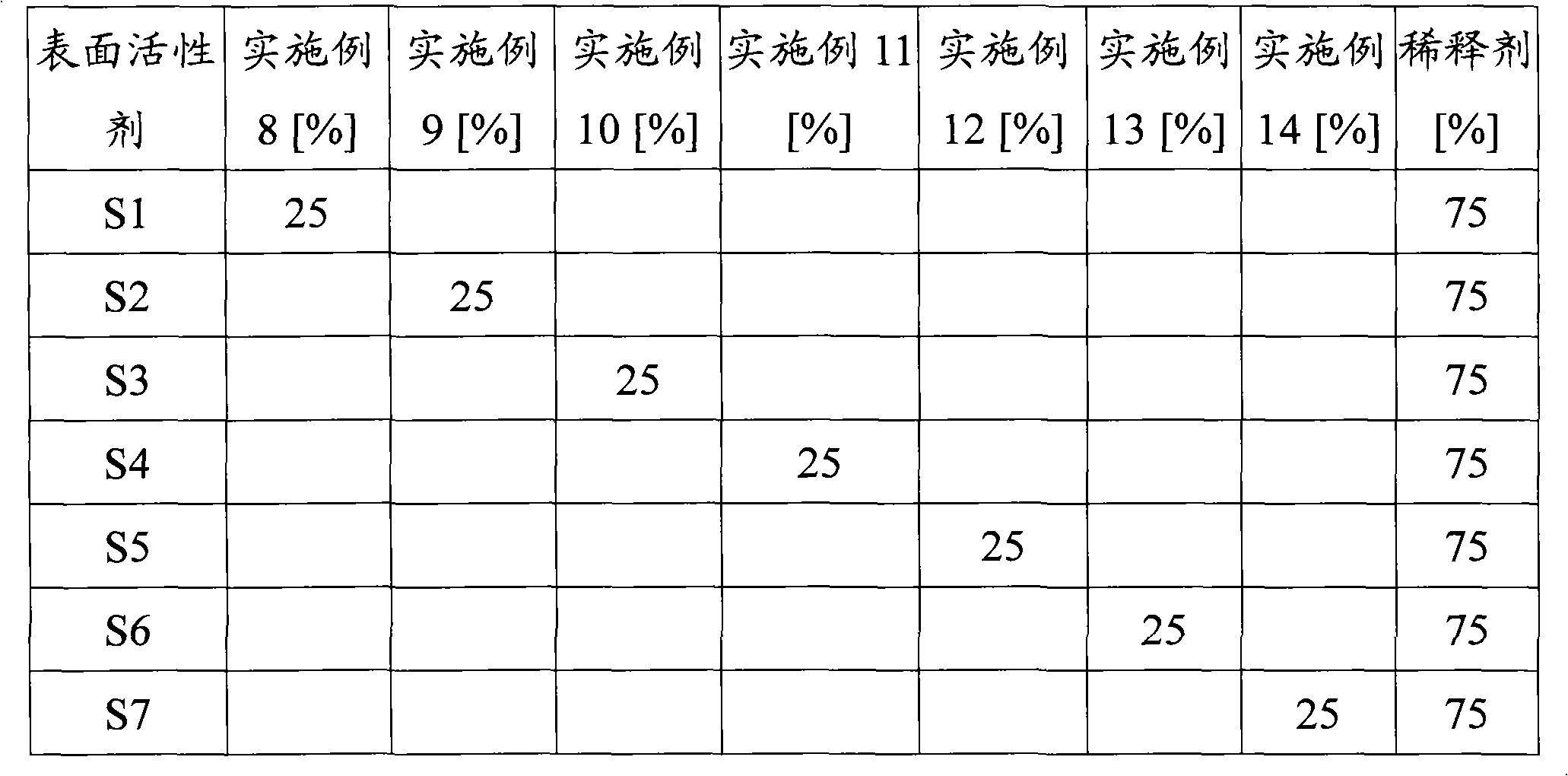
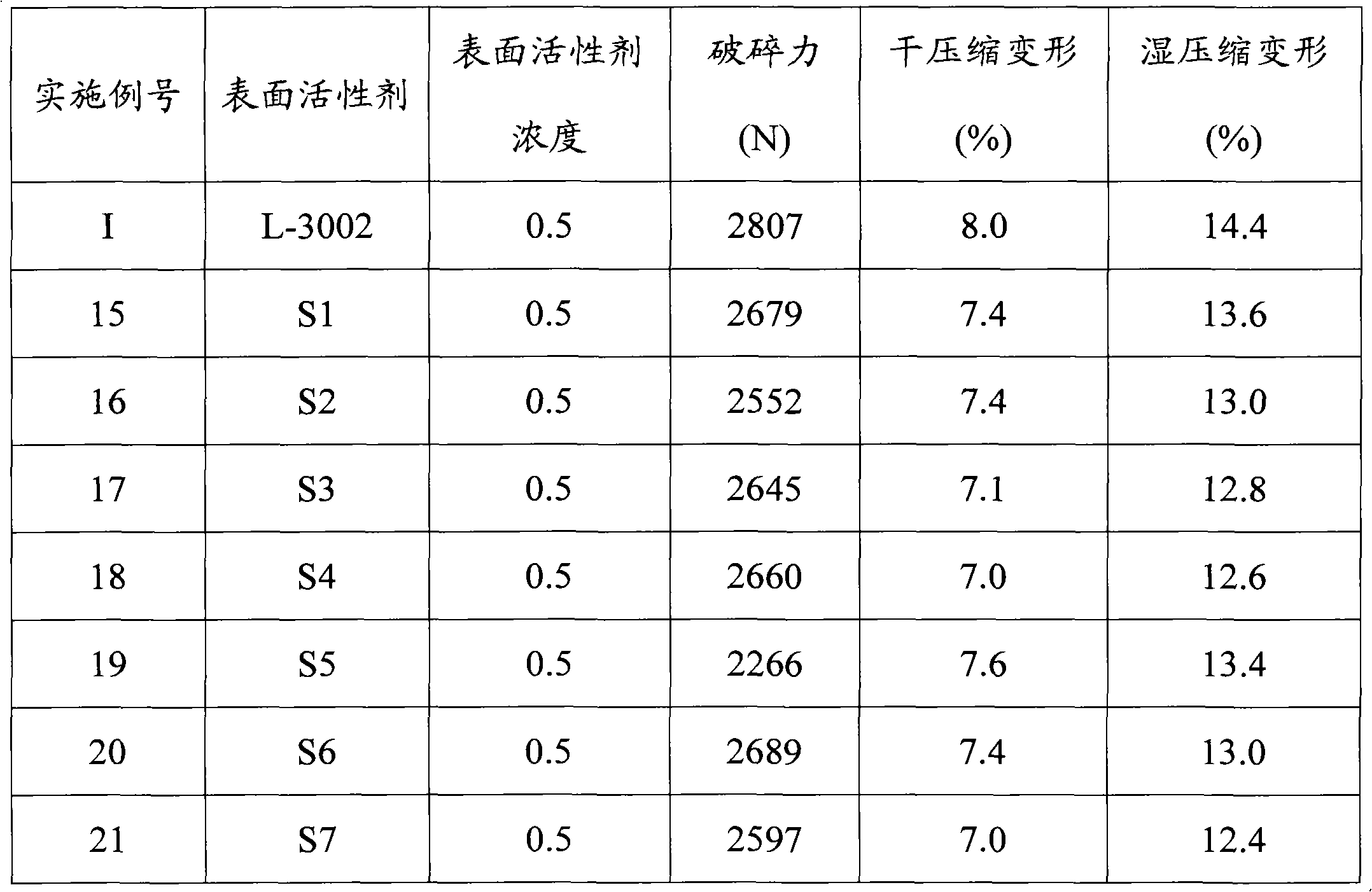
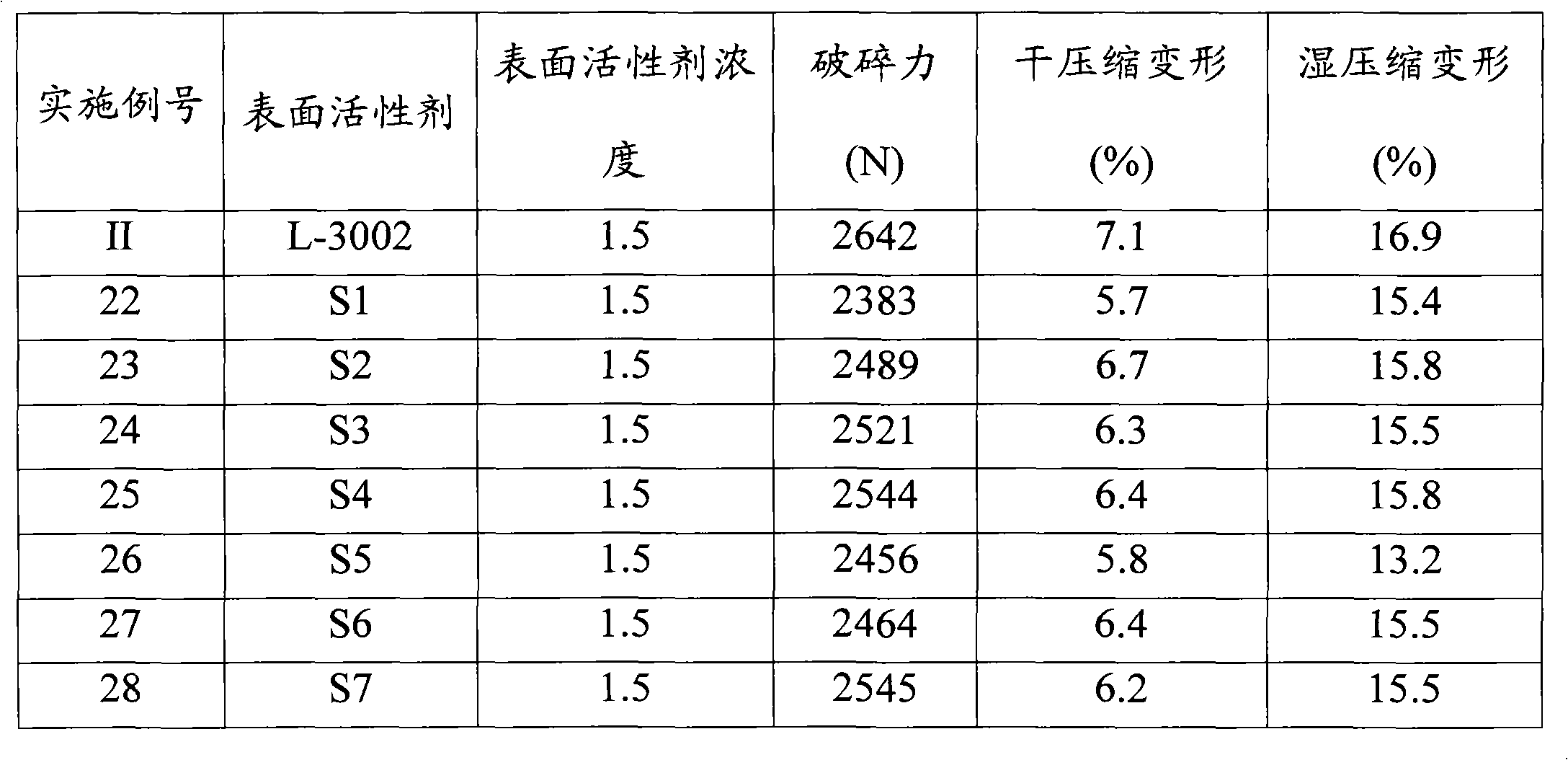
Organosilicone surfactant for producing flexible foam
A technology of surfactant and polyurethane foam, which is applied in the field of polyurethane foam forming composition, and can solve the problems such as deterioration of foam mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] 50.05 grams have the average general formula CH 2 =CHCH 2 Olefin-substituted polyoxyalkylenes of OH, 29.99 grams having the average general formula Me 3 SiO(MeHSiO) 1 SiMe 3 The mixture of organohydrogenpolysiloxane and nitrogen is stirred well and lightly sprayed. The flask was heated to 70°C. H in ethanol in an amount sufficient to provide 10 ppm Pt 2 PtCl 6 .6H2O solution was added to the mixture. The heat source was removed and the exothermic hydrosilylation reaction was allowed to begin until no further temperature rise was recorded. When the maximum temperature rose to 95 °C, 119.96 g of Me 3 SiO(MeHSiO) 1 SiMe 3 Add dropwise to the flask. The maximum temperature rose to 105°C and the flask was kept at this temperature for 1.5 hours. The residual SiH content was measured and observed to be below 0.1 g / cc, which means the hydrosilylation reaction is complete. The copolymer was cooled to 25°C and filtered.
Embodiment 2
[0137] 74.33 grams have the average general formula CH 2 =CHCH 2 O(CH 2 CH 2 O) 1.0 Olefin-substituted polyoxyalkylenes of H, 25.13 grams having the average general formula Me 3 SiO(MeHSiO) 1 SiMe 3 The mixture of organohydrogenpolysiloxane and nitrogen is stirred well and lightly sprayed. The flask was heated to 70°C. H in ethanol in an amount sufficient to provide 10 ppm Pt 2 PtCl 6 .6H2O solution was added to the mixture. The heat source was removed and the exothermic hydrosilylation reaction was allowed to begin until no further temperature rise was recorded. The highest temperature rose to 95 °C, and 100.54 g of Me 3 SiO(MeHSiO) 1 SiMe 3 Add dropwise to the flask. The maximum temperature was raised to 105°C and the mixture was allowed to stir for an additional 1.5 hours. The SiH content was measured and it was below 0.1 g / cc, which means the hydrosilylation reaction was complete. Allow the mixture to cool and cool the copolymer down to 25°C and filter.
Embodiment 3
[0139] The 110 grams have the average general formula CH 2 =CHCH 2 O(CH 2 CH 2 O) 3.45 Olefin-substituted polyoxyalkylenes of H, 18 grams having the average general formula Me 3 SiO(MeHSiO) 1 SiMe 3 The mixture of organohydrogenpolysiloxane and nitrogen is stirred well and lightly sprayed. The flask was heated to 80°C. H in ethanol in an amount sufficient to provide 10 ppm Pt 2 PtCl 6 .6H2O solution was added to the mixture. The heat source was removed and the exothermic hydrosilylation reaction was allowed to begin until no further temperature rise was recorded. The maximum temperature rose to 95 °C, and 72 g of Me 3 SiO(MeHSiO) 1 SiMe 3 Add dropwise to the flask. The maximum temperature was raised to 105°C and the reaction was kept stirring for 1.5 hours. SiH was measured and below 0.1 g / cc which means the hydrosilylation reaction was complete and the copolymer was cooled down to 25°C and filtered.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com