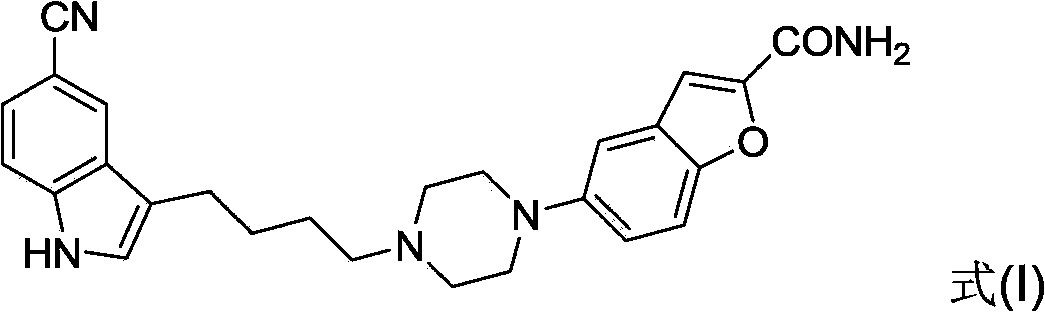
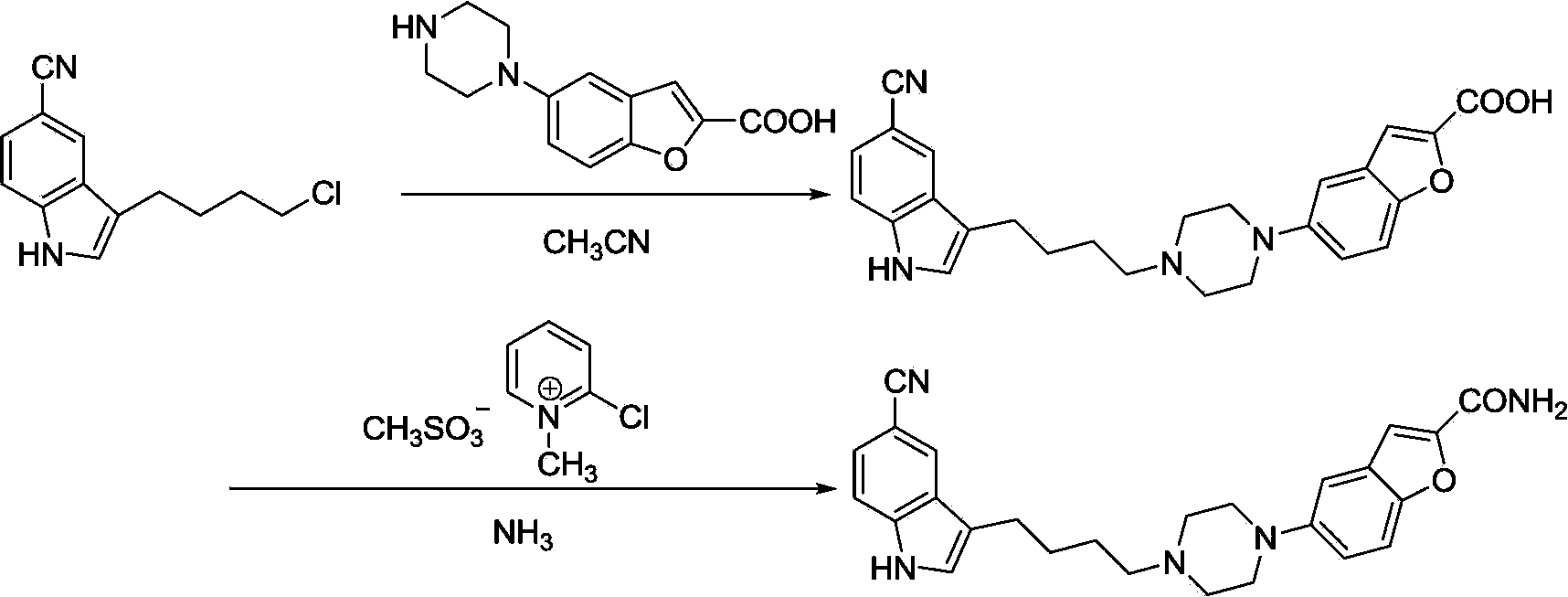
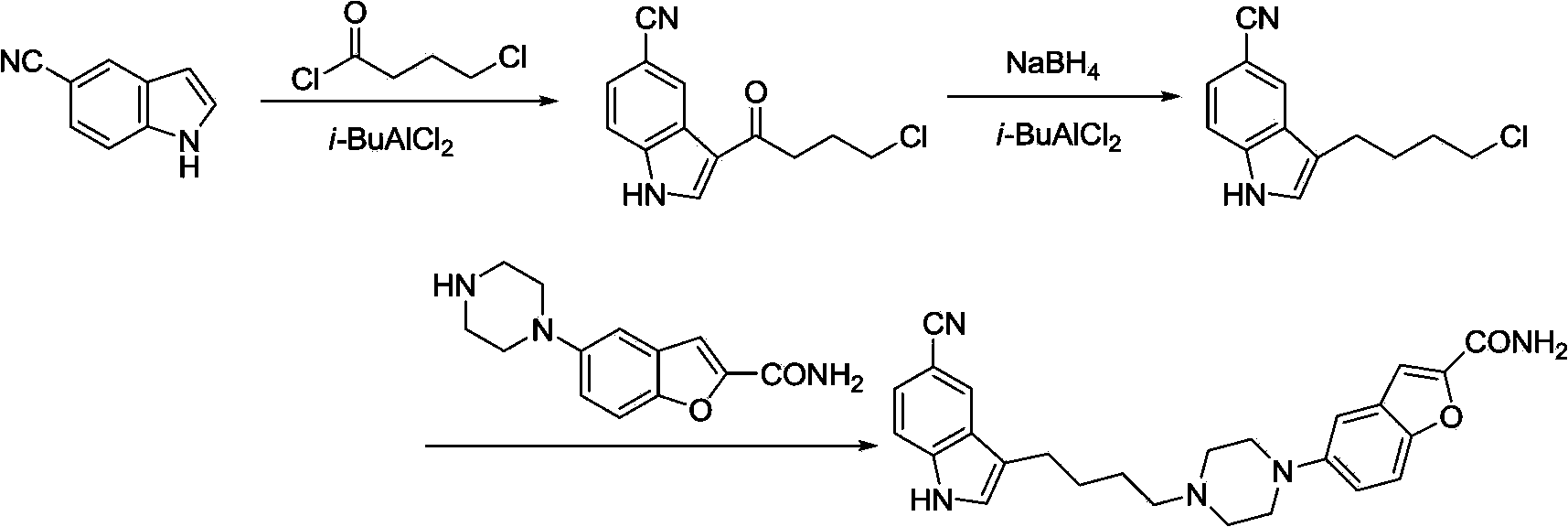
Method of preparing vilazodone and intermediate thereof
A compound and selected technology, applied in the direction of organic chemistry, can solve the problems of harsh Buchwald reaction conditions, not suitable for large-scale industrial production, and low reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097]
[0098] (1) Preparation of 5-(4-(3-butynyl)piperazin-1-yl)benzofuran-2-carboxamide
[0099] Add 24.5g (100mmol) of 5-(piperazin-1-yl)benzofuran-2-carboxamide, 57.1g (150mmol) of sodium phosphate dodecahydrate, 500ml tetrahydrofuran, and 125ml water in the reaction flask, and add dropwise at room temperature 4-Bromo-1-butyne 33.0g (250mmol), then heated to 65°C and stirred for 18h. The heat was turned off and the aqueous layer was separated while hot. The organic phase was evaporated to dryness under reduced pressure, and the solid was beaten with 500 ml of ethanol at room temperature, and filtered after two hours to obtain 25.6 g of a yellow solid, with a yield of 86.2%. MS-ESI[M+H] + :298
[0100] 1 H NMR: (400MHz, d 6 -DMSO)δ8.03(s,1H),7.61(s,1H),7.48(d,J=9.6Hz,1H),7.42(s,1H),7.16~7.18(m,2H),3.11(t ,J=4.8Hz,4H),2.79(s,1H),2.58(t,J=4.8Hz,4H),2.54(t,J=7.6Hz,2H),2.37(t,J=7.6Hz,2H ).
[0101] (2) 3-(4-(4-(2-carbamoylbenzofuran-5-yl)piperazin-1-yl)-1-butynyl)-5...
Embodiment 2
[0112]
[0113] (1) Preparation of 5-(4-(3-butynyl)piperazin-1-yl)benzofuran-2-carboxamide
[0114]Add 24.5g (100mmol) of 5-(piperazin-1-yl)benzofuran-2-carboxamide, 27.6g (200mmol) of sodium carbonate, 300ml tetrahydrofuran, and 200ml water into the reaction flask, and add 4-iodine dropwise at room temperature -1-butyne 36.0g (200mmol), then heated to 65°C and stirred for 18h. The heat was turned off and the aqueous layer was separated while hot. The organic phase was evaporated to dryness under reduced pressure, and the solid was beaten with 500 ml of ethanol at room temperature, and filtered after two hours to obtain 24.2 g of a yellow solid, with a yield of 81.5%.
[0115] (2) 5-(4-(4-(1-benzyl-5-cyanindol-3-yl)-3-but-ynyl)piperazin-1-yl)benzofuran-2-methyl Preparation of amides
[0116] Add 24.2 g (81.5 mmol) of 5-(4-(3-butynyl) piperazin-1-yl) benzofuran-2-carboxamide, 1-benzyl-3-iodoindole- 29.2g (81.5mmol) of 5-formonitrile, (1,1'-bis(diphenylphosphinoferrocene)...
Embodiment 3
[0120]
[0121] (1) Preparation of 5-(4-(3-butynyl)piperazin-1-yl)benzofuran-2-carboxamide
[0122] Add 24.5g (100mmol) of 5-(piperazin-1-yl)benzofuran-2-carboxamide, 30.3g (300mmol) of triethylamine, 300ml tetrahydrofuran, and 100ml water into the reaction flask, and add 4- Iodo-1-butyne 45.0g (250mmol), then heated to 80°C and stirred for 12h. The heat was turned off and the aqueous layer was separated while hot. The organic phase was evaporated to dryness under reduced pressure, and the solid was beaten with 500 ml of ethanol at room temperature, and filtered after one hour to obtain 22.9 g of a yellow solid, with a yield of 77.1%.
[0123] (2) 5-(4-(4-(5-cyano-1-(4-methoxybenzyl)indol-3-yl)-3-butynyl)piperazin-1-yl)benzene Preparation of furan-2-carboxamide
[0124] Add 22.9 g (77.1 mmol) of 5-(4-(3-butynyl) piperazin-1-yl) benzofuran-2-carboxamide in the reaction flask, 1-(4-methoxybenzyl) -3-bromoindole-5-carbonitrile 30.6g (90mmol), palladium acetate 0.224g (1mmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com