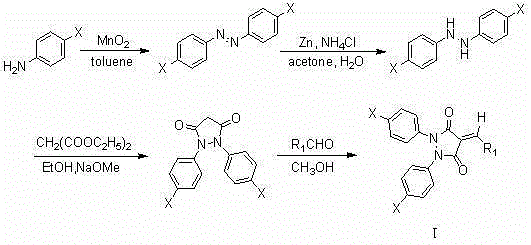
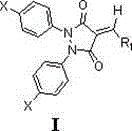
3,5-pyrazole diketone derivatives containing exocyclic double bond structural unit and its preparation method and use
A technology of pyrazolidinedione and pyrazolidinedione, which is applied in the field of pharmaceutical chemical synthesis to achieve the effects of high reaction yield, good market application prospects, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0015] Embodiment 11, the preparation of 2-two p-chlorophenyl-4-p-fluorobenzylidene-3,5-pyrazolidinedione
[0016] Add 2.008g (15.74mmol) p-chloroaniline and 50ml toluene into a 100ml three-neck flask, stir to dissolve, install the reflux tube and drying tube, add 6.892g (79.31mmol) MnO 2 , heated to 90°C, kept the temperature for 15 minutes, then raised the temperature to 118°C, and started to reflux. The TLC plate was spotted every 30 minutes to monitor the reaction progress (petroleum ether: chloroform = 2:1). Stop heating after the reaction is completed, and filter the reaction solution while hot with a sand core funnel covered with silica gel, and wash the filter cake twice with toluene. The filtrate was rotary evaporated to obtain an orange-yellow solid. Continue to wash the filter cake with the toluene evaporated by rotary evaporation, and repeat the operation until the filtrate after washing is colorless. The compound p-chloroazobenzene was obtained.
[0017] Add 0...
Embodiment 21
[0020] Example 21, Preparation of 2-two p-chlorophenyl-4-p-chlorobenzylidene-3,5-pyrazolidinedione
[0021] P-chlorobenzaldehyde was replaced by p-fluorobenzaldehyde, and the preparation method was the same as in Example 1 to obtain a yellow solid compound with a yield of 93.9%, m.p.204-206 ° C; 1 HNMR (400MHz, CDCl 3 )δ8.51(d, J =8.5Hz,2H),8.11(s,1H),7.51(d, J =8.5Hz,2H),7.40(dd, J =9.1,2.2Hz,2H),7.37(dd, J =9.1,2.2Hz,2H),7.31(d, J =8.9Hz,2H),7.31(d, J =8.9Hz,2H). 13 CNMR (101MHz, CDCl 3 )δ163.51, 161.84, 153.06, 141.23, 136.22, 134.87, 134.77, 132.47, 132.20, 130.73, 129.46, 129.26, 129.24, 123.74, 123.26, 116.89.HRMS(ESacIld.forC): m / zc 22 h 13 Cl 3 N 2 o 2 (M+H) + ,443.0121,found,443.0126.
Embodiment 31
[0022] Example 31, Preparation of 2-two-p-chlorophenyl-4-p-bromobenzylidene-3,5-pyrazolidinedione
[0023] Substitute p-bromobenzaldehyde for p-fluorobenzaldehyde, the preparation method is the same as in Example 1, and the yield of yellow solid compound is 94.2%, m.p.191-192°C; 1 HNMR (400MHz, CDCl 3 )δ8.39(d, J =8.6Hz,2H),8.07(s,1H),7.66(d, J =8.6Hz,2H),7.35(dd, J =9.1,2.2Hz,2H),7.35(dd, J=9.1,2.2Hz,2H),7.30(d, J =8.9Hz,2H),7.30(d, J =8.9Hz,2H). 13 CNMR (101MHz, CDCl 3 )δ161.41, 160.65, 152.11, 140.32, 135.13, 133.76, 133.24, 131.34, 131.11, 129.66, 128.44, 128.23, 128.16, 122.65, 122.14, 115.23. HRMS (ESIld.forC): m / zc 22 h 13 Cl 2 N 2 o 2 Br(M+H) + ,486.9616,found,486.9612.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com