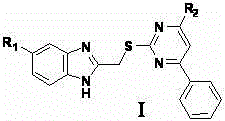
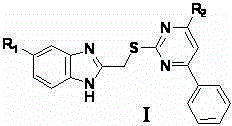
Pyrimidine derivatives with benzimidazole structural units as well as preparation method and application thereof
A technology of benzimidazole and structural unit, applied in the field of pharmaceutical chemical synthesis, achieving good market application prospects, mild reaction conditions, and reasonable synthesis design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
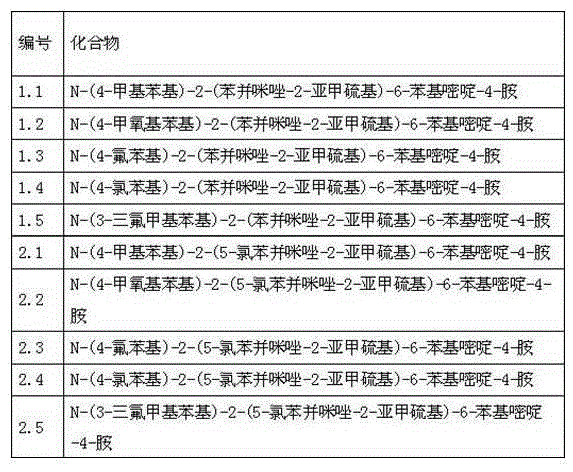
[0018] 1.1. N-(4-methylphenyl)-2-(benzimidazol-2-methylenethio)-6-phenylpyrimidin-4-amine
[0019] At 60°C, potassium hydroxide (644mg) and thiourea (874mg) were dissolved in 15ml absolute ethanol, then ethyl benzoyl acetate (1ml) was added and the temperature was raised to 90°C to reflux. Stop the reaction after 10 h, filter under reduced pressure while hot, wash the filter cake with ethanol (3×2ml), and dissolve the obtained solid in 50ml of distilled water after vacuum drying, adjust the pH to 6 with concentrated hydrochloric acid, and the solid yield is After washing with water and drying in vacuo, the compound 6-phenyl-2-thiouracil (960mg) was obtained;
[0020] At room temperature, dissolve potassium carbonate (276mg) in distilled water (6ml), then add 6-phenyl-2-thiouracil (408mg), stir to dissolve and heat up to 60°C; 2-chloromethylenebenzene The acetone solution of imidazole (2-chloromethylenebenzimidazole (322mg) dissolved in acetone (4ml)) was slowly a...
Embodiment 2
[0044] 2.1. N-(4-methylphenyl)-2-(5-chlorobenzimidazol-2-methylenethio)-6-phenylpyrimidin-4-amine
[0045] At 60°C, potassium hydroxide (644mg) and thiourea (874mg) were dissolved in 15ml absolute ethanol, then ethyl benzoyl acetate (1ml) was added and the temperature was raised to 90°C to reflux. Stop the reaction after 10 h, filter under reduced pressure while hot, wash the filter cake with ethanol (3×2ml), and dissolve the obtained solid in 50ml of distilled water after vacuum drying, adjust the pH to 6 with concentrated hydrochloric acid, and the solid yield is After washing with water and drying in vacuo, the compound 6-phenyl-2-thiouracil (960mg) was obtained;
[0046] At room temperature, dissolve potassium carbonate (80mg) in distilled water (6ml), then add 6-phenyl-2-thiouracil (408mg), stir to dissolve and heat up to 60°C; 5-chloro-2-chloro The acetone solution of methylenebenzimidazole (5-chloro-2-chloromethylenebenzimidazole (400mg) dissolved in acetone (4ml)) was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com