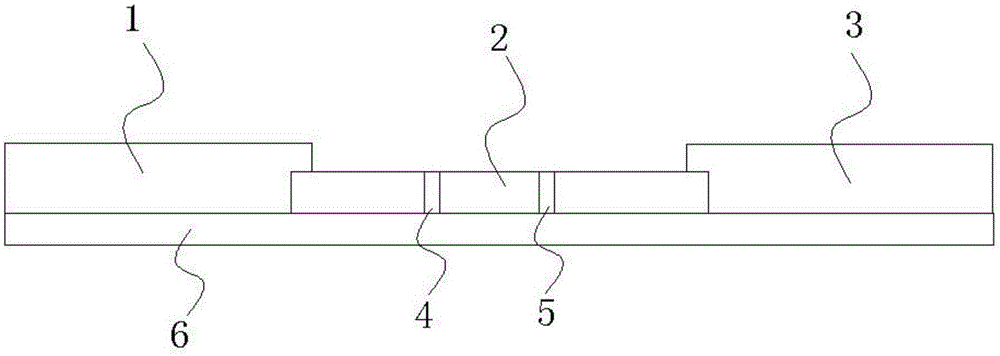
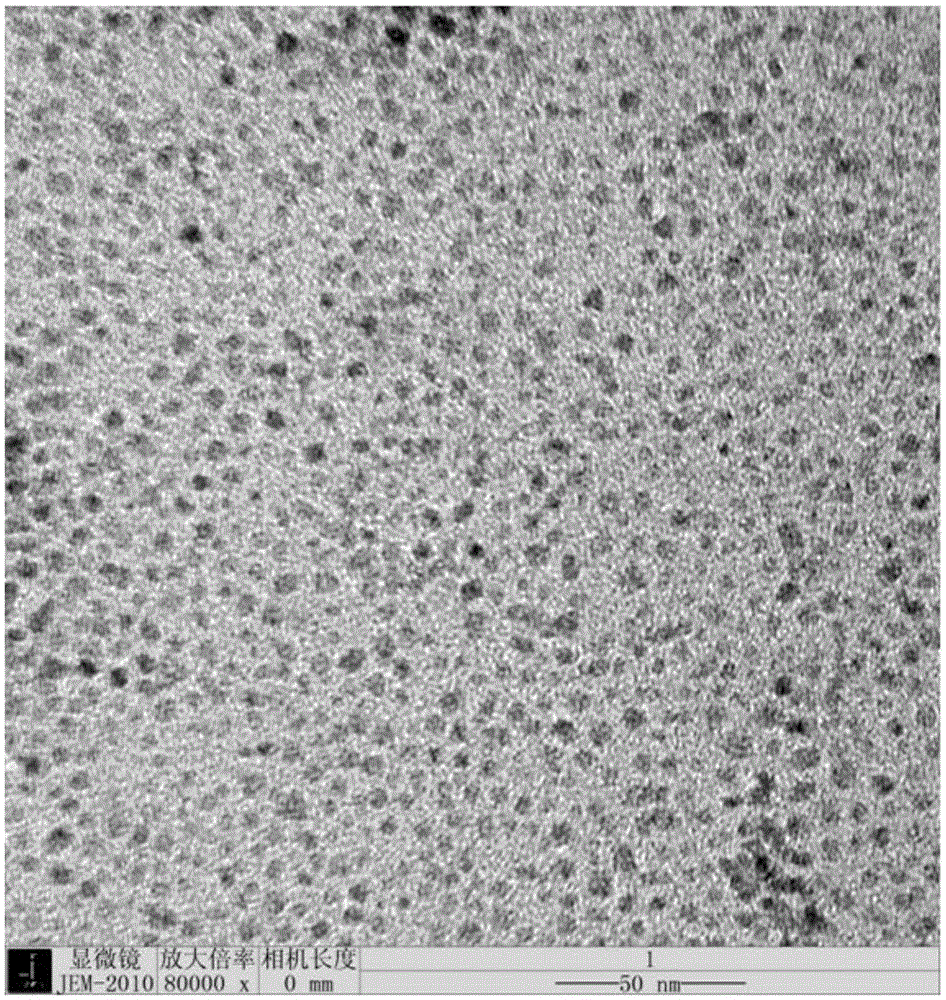
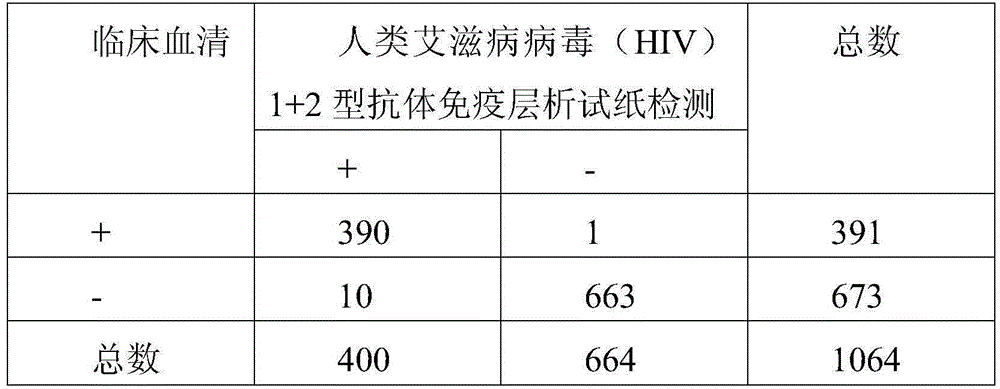
Immunochromatographic test paper for detecting human AIDS virus antibody and preparation method thereof
An immunochromatographic test strip, HIV technology, applied in coatings, measuring devices, analytical materials, etc., can solve the problems of inability to provide sufficient magnetic resonance signals, easy polymerization of magnetic beads, and long color development time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] (1) Preparation of recombinant protein A labeled with superparamagnetic composite particles
[0053] Superparamagnetic Fe with a particle size of 100nm, a particle size deviation of 15%, a magnetic saturation intensity of 40emu / g, a corresponding external magnetic field response speed of 20 seconds, and a surface carboxyl content of 80μmol / g was used. 3 o 4 Nanoparticles.
[0054] The specific method is: take 2.5mg of the above-mentioned superparamagnetic Fe 3 o 4 Nanoparticles were washed with MES buffer solution with a concentration of 0.1 mol and a pH of 4.7, and separated and enriched with a 0.4T magnetic rack, then resuspended with 1 ml of the above-mentioned MES buffer solution, and then added 0.96 mg of EDC and 2.17 mg of NHS, Mix well, react at a reaction temperature of 37° C. for 0.5 hour, and then wash with borax buffer solution with a concentration of 50 mmol and a pH of 8.5 to obtain 2.5 mg of activated magnetic particles.
[0055] Take 1.5 mg of recombi...
Embodiment 2
[0061] (1) Preparation of recombinant protein A labeled with superparamagnetic composite particles
[0062] Superparamagnetic Fe with a particle size of 60nm, a particle size deviation of 10%, a magnetic saturation intensity of 80emu / g, a corresponding external magnetic field response speed of 100 seconds, and a surface carboxyl content of 50μmol / g was used. 3 o 4 Nanoparticles.
[0063] The specific method is: take 2.5mg of the above-mentioned superparamagnetic Fe 3 o 4 Nanoparticles were washed with MES buffer solution with a concentration of 0.1 mol and a pH of 4.7, and separated and enriched with a 0.4T magnetic rack, then resuspended with 1 ml of the above-mentioned MES buffer solution, and then added 0.96 mg of EDC and 2.17 mg of NHS, Mix well, react at a reaction temperature of 35° C. for 40 minutes, and then wash with a borax buffer solution with a concentration of 50 mmol and a pH of 8.5 to obtain 2.5 mg of activated magnetic particles.
[0064] Take 1.5 mg of rec...
Embodiment 3
[0069] (1) Preparation of recombinant protein A labeled with superparamagnetic composite particles
[0070] Superparamagnetic Fe with a particle size of 300nm, a particle size deviation of 20%, a magnetic saturation intensity of 30emu / g, a corresponding external magnetic field response speed of 100 seconds, and a surface carboxyl content of 300μmol / g was used. 3 o 4 Nanoparticles.
[0071] The specific method is: take 2.5mg of the above-mentioned superparamagnetic Fe 3 o 4 Nanoparticles were washed with MES buffer solution with a concentration of 0.1 mol and a pH of 4.7, and separated and enriched with a 0.4T magnetic rack, then resuspended with 1 ml of the above-mentioned MES buffer solution, and then added 0.96 mg of EDC and 2.17 mg of NHS, Mix well, react at a reaction temperature of 37° C. for 20 minutes, and then wash with a borax buffer solution with a concentration of 50 mmol and a pH of 8.5 to obtain 2.5 mg of activated magnetic particles.
[0072] Take 1.5 mg of r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com