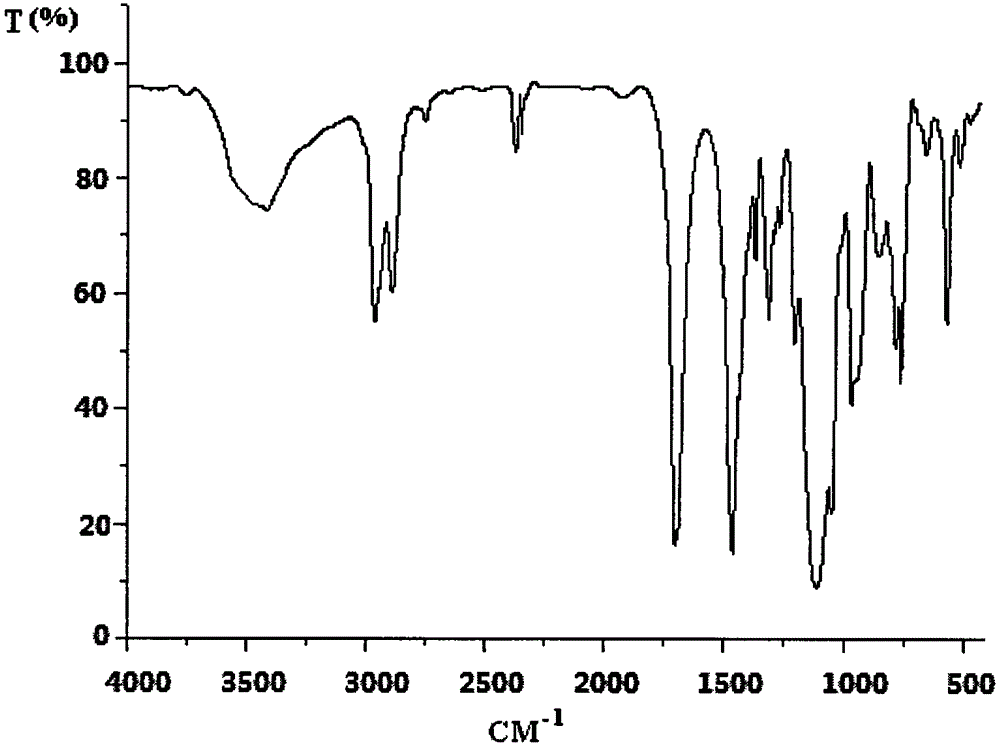
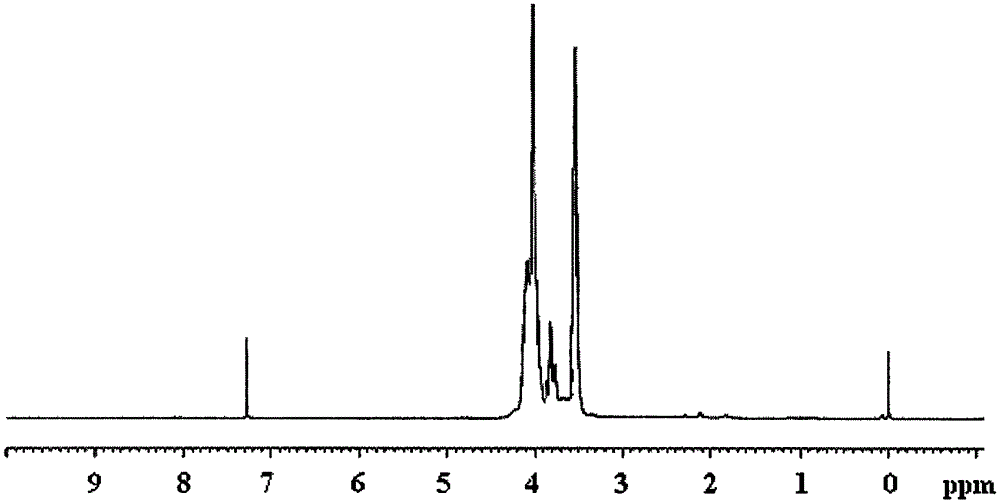
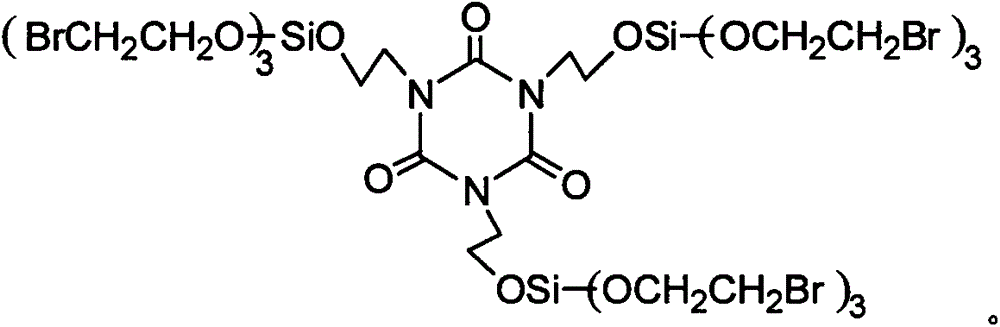
Flame retardant cyclo-bromoethyl trisilicate compound and preparation method thereof
A flame retardant and compound technology, which is applied in the field of flame retardant Seike trisilicate bromoethyl ester compound and its preparation field, can solve the problems of flammability, fire, life and property safety threats of synthetic polymer materials, etc., and it is difficult to improve The effect of low dispersion and activity, low production cost and high synergistic flame retardant efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 In a 250ml four-neck flask equipped with a stirrer, a thermometer and a high-efficiency reflux condenser, and a drying tube on the upper mouth of the condenser, replace the air in the bottle with nitrogen, add 20ml of dichloroethane and 8.5 g (5.67ml, 0.05mol) of silicon tetrachloride, under stirring, cooled with a cold water bath to lower the temperature of the reaction system below 20°C, add 6.25g (3.55ml, 0.05mol) of bromoethanol dropwise, and the dropwise addition process is controlled The reaction temperature is not higher than 30°C. After dripping, raise the temperature to 40°C, and keep it warm for 2 hours; after the HCl gas is released, dissolve 4.358g (0.0167mol) Cycla in 50ml of dichloroethane dropwise into the In the four-necked flask, the reaction temperature was controlled by the dropping rate to not be higher than 60°C. After the dropping, the temperature was raised to 80°C, and the reaction was carried out for 9 hours; ml, 0.101mol) of bromoetha...
Embodiment 2
[0031]Embodiment 2 In the 25Oml four-neck flask that agitator, thermometer and high-efficiency reflux condensation tube are equipped with, and drying tube is housed on the top of condensation tube, replace the air in the bottle with nitrogen, add 2Oml dioxane and 8.5 g (5.67ml, 0.05mol) of silicon tetrachloride, under stirring, cooled with a cold water bath to lower the temperature of the reaction system below 20°C, add 6.25g (3.55ml, 0.05mol) of bromoethanol dropwise, and the dropwise addition process is controlled The reaction temperature is not higher than 30°C. After dripping, raise the temperature to 40°C, and keep it warm for 2 hours; after the HCl gas is released, dissolve 4.358g (0.0167mol) Cycla in 50ml of dioxane dropwise into the four In the open flask, the reaction temperature is controlled by the dropping rate not to be higher than 60°C. After the drop, the temperature is raised to 85°C and reacted for 8 hours; after the HCl gas is released, the system is cooled to...
Embodiment 3
[0032] Embodiment 3 In the 250ml four-necked flask that stirrer, thermometer and high-efficiency reflux condenser are equipped with, and drying tube is housed on the condenser upper mouth, replace the air in the bottle with nitrogen, add 20ml acetonitrile and 8.5g (5.67 ml, 0.05mol) of silicon tetrachloride, under stirring, cool with a cold water bath to reduce the temperature of the reaction system to below 20°C, add 6.25g (3.55ml, 0.05mol) of bromoethanol dropwise, and control the reaction temperature during the dropwise addition Not higher than 30°C. After dropping, raise the temperature to 40°C, and keep it warm for 2 hours; after the HCl gas is released, dissolve 4.358g (0.0167mol) Cycla in 50ml of acetonitrile into the four-necked flask, Control the reaction temperature not higher than 60°C with the dropping speed, raise the temperature to 80°C after dropping, react for 9 hours, after the HCl gas is released, cool the system down to below 40°C, add dropwise 15.0g (8.51ml,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flash point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com