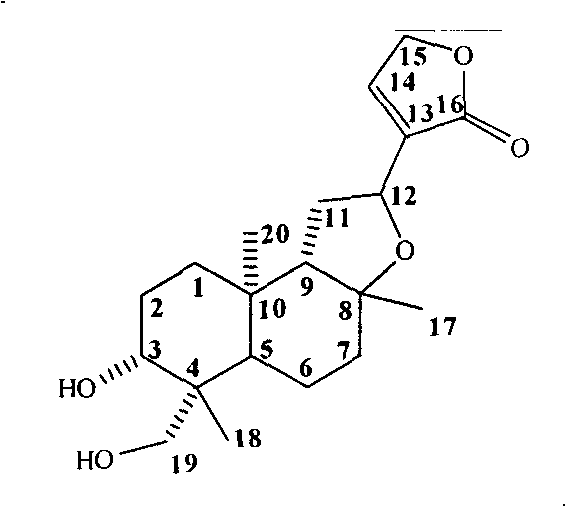
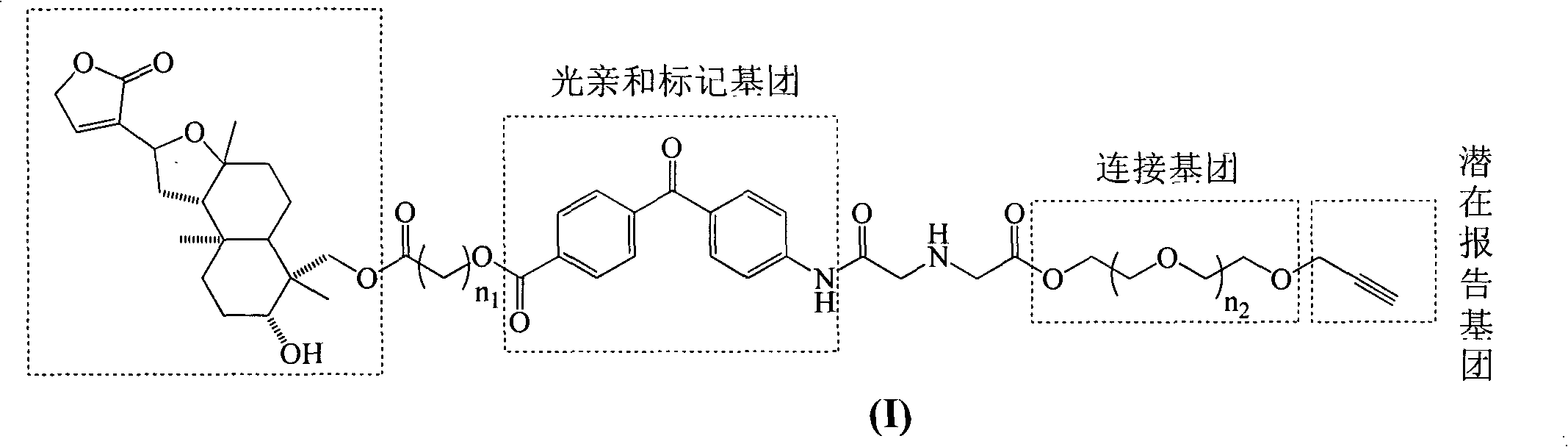
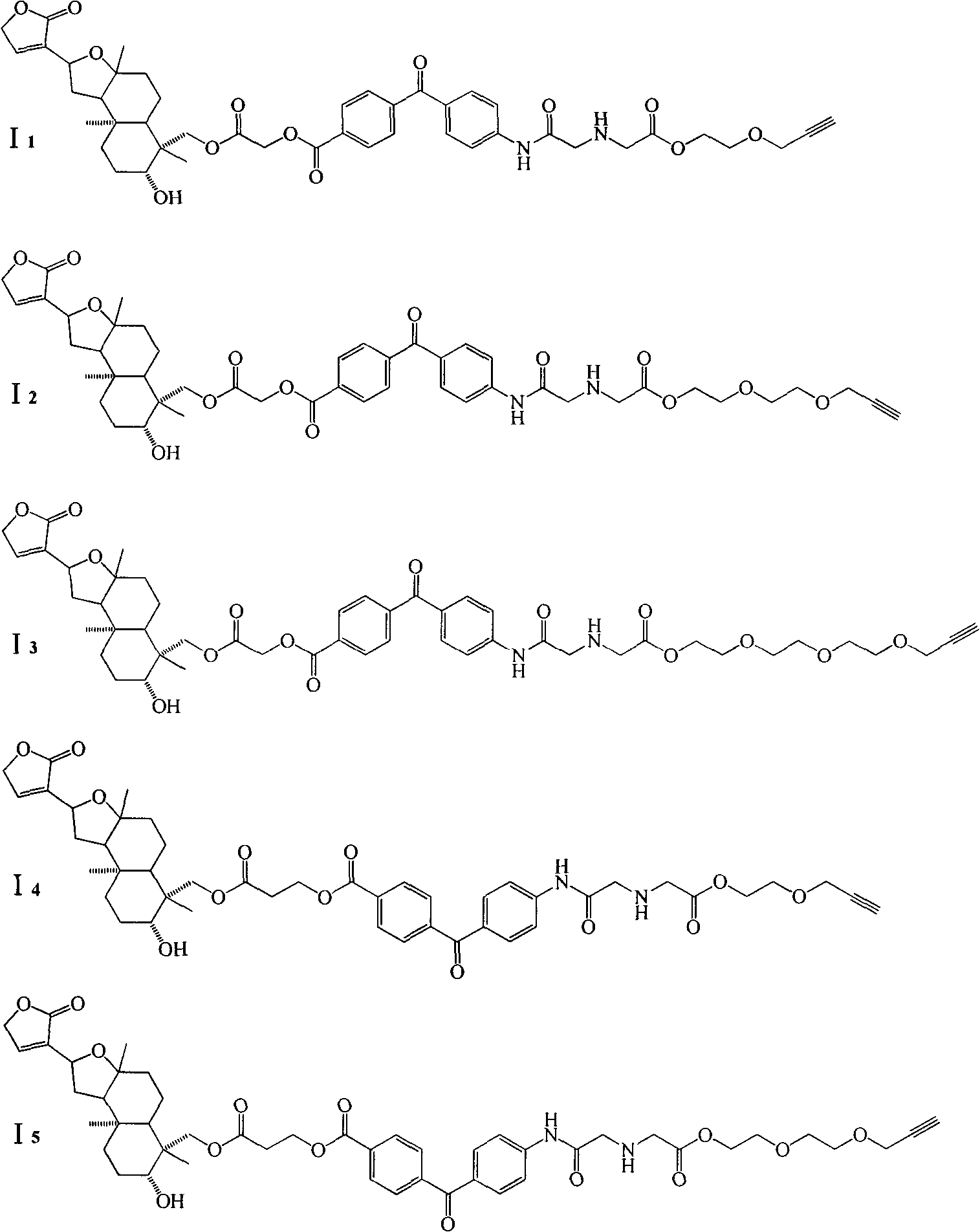
Isoandrographolide photoaffinity labeling molecular probe, preparation method and pharmaceutical composition of molecular probe
A technology of andrographolide and marker molecule, which can be used in drug combinations, medical preparations containing active ingredients, antipyretics, etc., and can solve the problem that the mechanism of action of the target protein is not fully elucidated.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of isoandrographolide II
[0044] Dissolve 5.0 g of andrographolide in 80 mL of concentrated hydrochloric acid, stir magnetically at room temperature until completely dissolved, and place overnight. After the reaction was completed, the reaction solution was poured into a beaker filled with ice-cold 200 mL saturated saline solution, and a saturated sodium carbonate solution was slowly added with stirring until the pH was about 7.0. Extracted with ethyl acetate (100 mL×8), washed with saturated brine (100 mL×3), dried over anhydrous sodium sulfate, concentrated by filtration, and the crude product was recrystallized from absolute ethanol to obtain 3.0 g of white solid, yield 60%. 1 H-NMR (CDCl 3 , 400MHz) δ: 7.29 (1H, overlapped signal, H-14), 4.82 (2H, s, H-15), 4.71 (1H, t, J=20.0Hz, H-12), 4.27 (1H, d, J=12.0Hz, H-19a), 3.47(1H, dd, J=4.0Hz, 8.0Hz, H-3), 3.38(1H, d, J=12.0Hz, H-19b), 2.48~2.42(4H , -OH, 11), 2.25~2.19(2H, 7a, 9), 2.07~1.99(1H, H-7b), 1....
Embodiment 2
[0046] Compound III 1 preparation of
[0047] 1.0g II was dissolved in 130mL dichloromethane, and 0.515g K was added 2 CO 3 , stirred magnetically at room temperature for 30 min, then slowly added 0.638 g of bromoacetyl bromide dropwise, and reacted at room temperature for 3 h. After the reaction was completed, it was washed with saturated brine (50mL×3), dried over anhydrous magnesium sulfate, and separated by silica gel column chromatography (V 石油醚 :V 乙酸乙酯 =1:1), to obtain 1.145g compound III 1 , yield 85%. 1 H-NMR (CDCl 3 , 400MHz) δ: 7.29 (1H, overlapped signal, H-14), 4.83 (2H, s, H-15), 4.73 (1H, t, J=20.0Hz, H-12), 4.56 (1H, d, J = 12.0Hz, H-19a), 4.26 (1H, d, J = 12.0Hz, H-19b), 3.86 (2H, s, 19-CO CH 2 Br), 3.36 (1H, dd, J=8.0Hz, 4.0Hz, H-3), 2.51~2.45 (1H, H-11a), 2.26~2.19 (5H, -OH, 7a, 9, 11b), 2.08 ~2.02(1H, H-7b), 1.83~1.50(7H, H-1, 2, 5, 6), 1.23(3H, s, H-17), 1.13(3H, overlapped-signal, H-18) , 1.00 (3H, overlapped-signal, H-20).
[0048] Compound II...
Embodiment 3
[0057] Compound V 1 preparation of
[0058] 0.5 g compound III 1 Dissolve in 50mL acetone, add 0.2g K 2 CO 3 , stirred magnetically at room temperature for 30 min, then gradually added 0.31 g of photoaffinity labeling group IV, and stirred magnetically at room temperature for 4 h. After the reaction is complete, pour the reaction solution into a beaker containing 100 mL of saturated sodium bicarbonate solution, extract with dichloromethane (100 mL×3), and then use saturated sodium bicarbonate solution (100 mL×3), saturated saline (100 mL×3 ) washing, dried over anhydrous magnesium sulfate, separated by silica gel column chromatography (V 石油醚 :V 乙酸乙酯 =1:1), to obtain 0.610g compound V 1 , yield 91%. 1 H-NMR (CDCl 3 , 400MHz) δ: 8.17 (2H, d, J = 8.0Hz, ph), 7.76 (2H, d, J = 8.0Hz, ph), 7.69 (2H, d, J = 8.0Hz, ph), 7.27 (1H , overlapped-signal, H-14), 6.68 (2H, d, J=8.0Hz, ph), 4.88 (2H, d, J=2.5Hz, 19-CO CH 2 ), 4.79 (2H, s, H-15), 4.69 (1H, t, J=16.0Hz, H-12), 4.61 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com