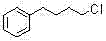Preparation method of 1-chloro-4-phenyl butane
A technology of phenylbutane and dichlorobutane, applied in the field of preparation of pharmaceutical intermediates, can solve the problem of high cost and achieve the effects of low cost, convenient operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Put 1,4-dichlorobutane (1000g, 7.87mol, 6.0eqv.) into a 2000ml four-necked flask, and add anhydrous aluminum chloride (8.7g, 0.065mol, 0.05eqv.) under nitrogen protection , Mechanical stirring, ice water cooling, at 3 oC Begin to add benzene (102.5g, 1.31mol) dropwise, add it in 5 hours, then continue to stir for 10 minutes, add 150g of pure water dropwise in 8 minutes, stir for 30 minutes and then stand at room temperature, separate the upper layer (water layer, containing chlorine Aluminium), washed three times to pH 6~7, the organic layer is rectified under normal pressure (tower height is 100cm), and a small amount of benzene (21g) is recovered. It can be used after drying through molecular sieve, containing 1~2% 1,4-dichlorobutane Alkane does not affect the application, bp. 77~80 oC ), and then collect the second fraction 1,4-dichlorobutane (828 grams, bp. 152~154 oC , Can be applied after being dried by molecular sieve), and then the collected fraction is tetrali...
Embodiment 2
[0027] 17.3g (0.13mol, 0.10eqv.) of anhydrous aluminum chloride, other operations are the same as in Example 1, and the yield is 69%.
Embodiment 3
[0029] 1,4-Dichlorobutane 500g (3.94mol, 3.0eqv.), other operations are the same as in Example 1, the yield is 64%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com