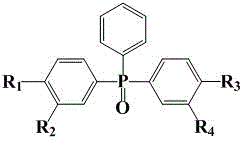
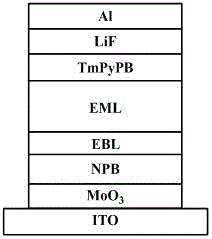
Phosphorescent host material, its preparation method and application, and electrophosphorescent light-emitting device
A light-emitting device and electrophosphorescence technology, which is applied in the field of electrophosphorescence light-emitting devices, its preparation, and phosphorescent host materials, can solve the problems of low device efficiency, low electron mobility, and large device efficiency roll-off, etc., and achieve improved device performance. Efficiency, improvement of triplet energy level, and improvement of electron mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
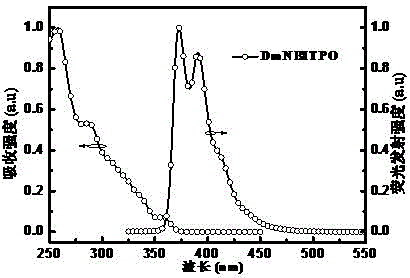
[0041] For DmNBITPO, its preparation method is as follows:
[0042] Step a: Dissolve 9,10-phenanthrenequinone (3.0mmol), benzaldehyde (3.0mmol), m-bromoaniline (10.0mmol) and ammonium acetate (12.0mmol) in acetic acid (200ml), heat to 120°C to reflux 48 hours, then cooled to room temperature, added water to precipitate a solid, filtered, washed the resulting solid with water, and dried to obtain m-bromo- N - phenylphenanthroimidazole intermediate;
[0043] Step b: Dissolve the m-bromo-N-phenylphenanthroimidazole intermediate (1.0 mmol) obtained in step a in THF (tetrahydrofuran) (50 ml), cool to -80 ° C, add phenyl phosphorus dichloride ( 0.5mmol), continue to react for 4 hours, be warmed up to room temperature for 12 hours, add water to quench the reaction, add 30%H at the same time 2 o 2 Aqueous solution (5.0ml) was oxidized to give bis(3-(1-phenyl-2-N-phenylphenanthroimidazolyl)triphenylphosphine oxide (DmNBITPO); yield: 65%. Mass spectrum (mass spectrum) MS (APCI): cal...
Embodiment 2
[0045] For DpNBITPO, its preparation method is as follows:
[0046] Step a: Dissolve 9,10-phenanthrenequinone (3.0mmol), benzaldehyde (3.0mmol), p-bromoaniline (10.0mmol) and ammonium acetate (12.0mmol) in acetic acid (200ml), heat to 120°C to reflux 48 hours, then cooled to room temperature, added water to precipitate solids, filtered, washed the resulting solids with water, and dried to obtain p-bromo- N - phenylphenanthroimidazole intermediate;
[0047] Step b: the p-bromo- N -Phenylphenanthroimidazole intermediate (1.0mmol) was dissolved in THF (tetrahydrofuran) (50ml), cooled to -80°C, added phenylphosphorous dichloride (0.5mmol), continued the reaction for 4 hours, and warmed to room temperature for 12 hour, add water to quench the reaction, add 30% H at the same time 2 o 2 Aqueous solution (5.0ml) was oxidized to give bis(4-(1-phenyl-2-N-phenylphenanthroimidazolyl)triphenylphosphine oxide (DpNBITPO); yield: 74%. Mass spectrum (mass spectrum) MS (APCI): calcdforC 6...
Embodiment 3
[0049] For DmBITPO, its preparation method is as follows:
[0050] Step a: Dissolve 9,10-phenanthrenequinone (3.0mmol), m-bromobenzaldehyde (3.0mmol), aniline (10.0mmol) and ammonium acetate (12.0mmol) in acetic acid (200ml), heat to 120°C to reflux 48 hours, then cooled to room temperature, added water to precipitate a solid, filtered, washed the obtained solid with water, and dried to obtain the N-m-bromophenyl-2-phenylphenanthroimidazole intermediate;
[0051] Step b: Dissolve the N-m-bromophenyl-2-phenylphenanthroimidazole intermediate (1.0 mmol) obtained in step a in THF (tetrahydrofuran) (50 ml), cool to -80 ° C, add phenyl di Phosphorous chloride (0.5mmol), continue to react for 4 hours, warm up to room temperature for 12 hours, add water to quench the reaction, add 30% H 2 o 2 Aqueous solution (5.0ml) was oxidized to give bis(3-(N-phenyl-1-N-phenylphenanthroimidazolyl)triphenylphosphine oxide (DmBITPO); yield: 74%. Mass spectrum (mass spectrum) MS (APCI): calcdforC6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com