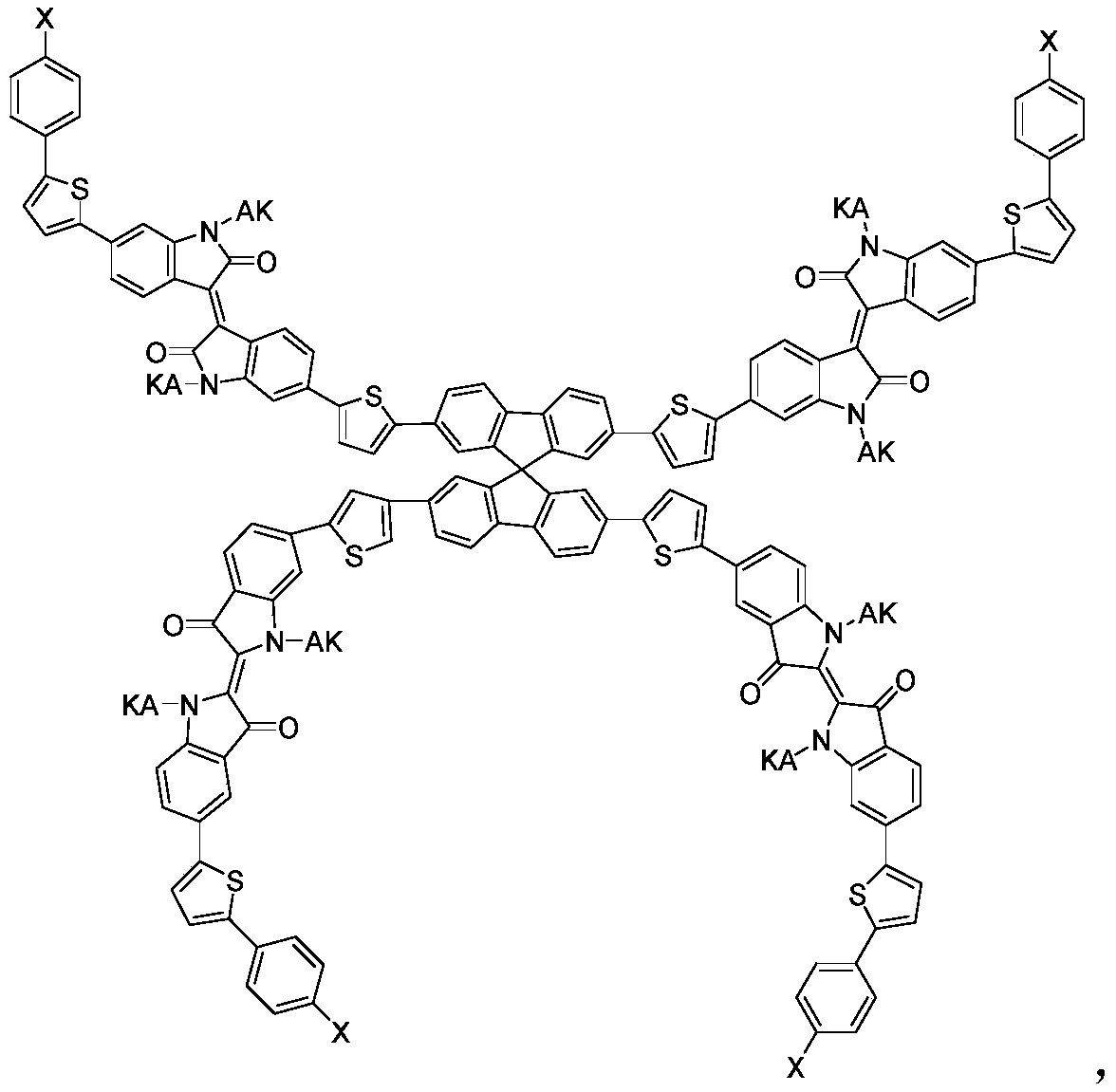
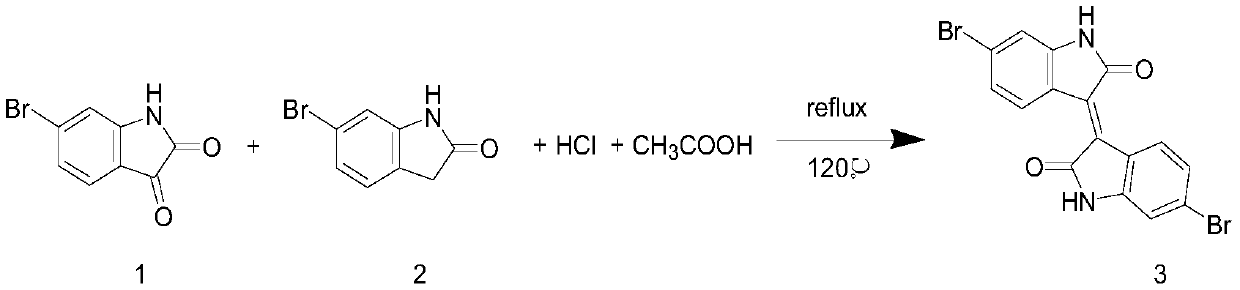A kind of acceptor material of organic solar cell and preparation method thereof
A technology for solar cells and acceptor materials, applied in the field of isoindigo derivative organic solar cell acceptor materials and their preparation, can solve the problems of long energy repayment time, complicated preparation procedures, easy to produce pollution, etc. Capability, high carrier mobility, solubility improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 6-bromooxindole (compound 1, 500 mg, 2.36 mmol), 6-bromoisatin (compound 2, 533 mg, 2.36 mmol) and AcOH (15 mL) into a 50 ml one-necked flask, and add concentrated HCl to the suspension solution (0.1ml), heated to 100°C and refluxed for 24 hours. After the mixture was cooled, it was filtered, and the solid matter was washed with water, EtOH and AcOEt respectively, followed by vacuum drying to obtain 951 mg of brown 6,6-dibromoisoindole (Compound 3), with a yield of 95%. The reaction process is as follows:
[0034]
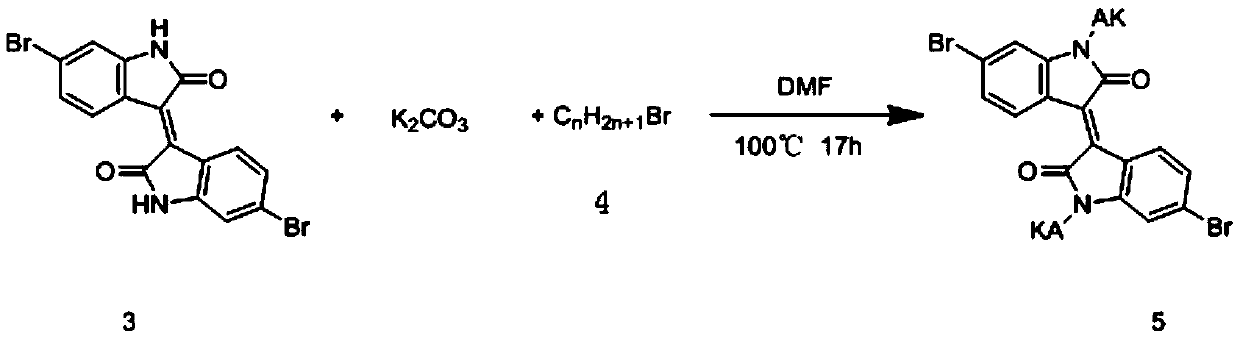
[0035] Under the protective atmosphere of inert gas in a vacuum environment, add 6,6-dibromoisoindole (compound 3, 1.0g, 2.93mmol), freshly dried potassium carbonate (2.43g, 17.59mmol) and anhydrous DMF into a 100ml three-necked flask (20 mL), followed by the addition of 1-bromo-2-ethylhexane (1.70 g, 8.79 mmol) to the resulting suspension. The mixture was heated to 100 °C and stirred for 15 hours, then poured into water (500 mL), and the organic ph...
Embodiment 2
[0054] The synthesis process of compound 3 was as described above.
[0055] Under the protective atmosphere of inert gas in a vacuum environment, add 6,6-dibromoisoindole (compound 3, 1.0g, 2.38mmol), freshly dried potassium carbonate (2.43g, 17.59mmol) and anhydrous DMF to a 100ml three-necked flask (25 mL), then 1-bromo-n-hexyl (compound 4, 0.98 g, 5.95 mmol) was added to the resulting suspension. The mixture was heated to 100 °C and stirred for 15 hours, then poured into water (500 mL), and the organic phase was washed with CH 2 Cl 2 Extracted, washed with brine, washed with MgSO 4 After drying and removal of the solvent under reduced pressure, the dark red solid was purified by silica chromatography with (CH 2 Cl 2 : hexane=2:3, volume ratio) to obtain 1.26 g of brown solid (compound 5.1) with a yield of 90.7%. The reaction process is as follows:
[0056]
[0057] Add toluene (47mL), triphenylphosphine palladium (Pd(PPh 3 ) 4 ) (0.26g, 13.2mmol) and compound 5.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com