Method for synthesizing 1,4-naphthoquinone through catalyzing ionic liquid
A technology of ionic liquid and naphthoquinone, which is applied in the field of preparation of carbocyclic compounds, can solve the problems of high price and environmental pollution, achieve high selectivity, good separation, and solve the effects of poor reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
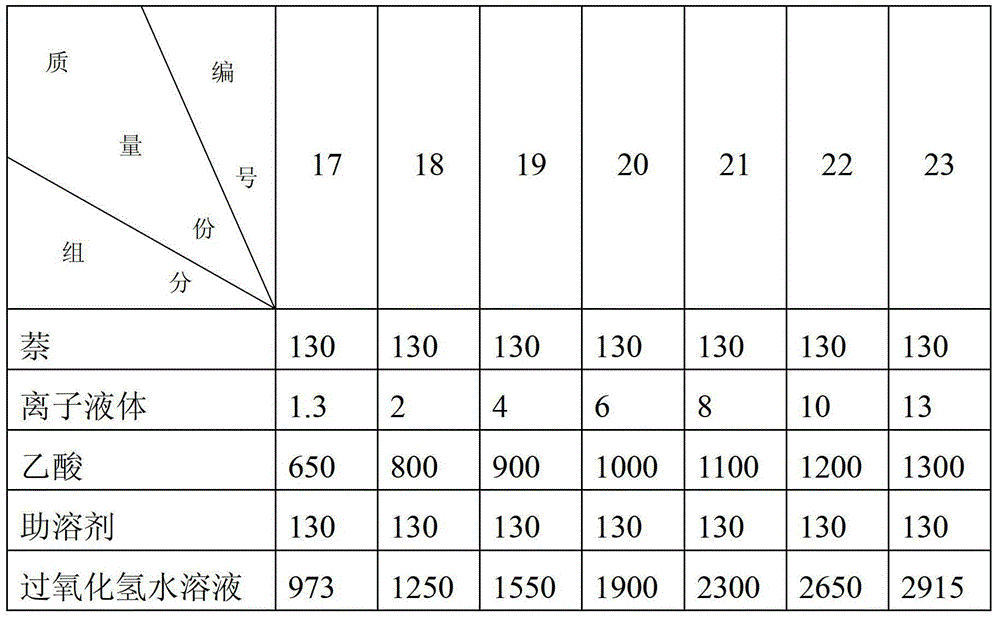
[0021] 13kg naphthalene, 0.13kg ionic liquid [Bu 3 NH][HSO 4 ], 70kg of acetic acid and 13kg of co-solvent petroleum ether were added to the reactor, and the temperature was raised to 90°C under stirring, and 134kg of aqueous hydrogen peroxide solution with a concentration of 30% by mass was added dropwise within 1.5 to 2.5 hours. , filtered out the insoluble precipitate, and the filtrate was extracted with cyclohexane, washed with water, dried, and then distilled under reduced pressure to obtain 7.49kg of 1,4-naphthoquinone with a yield of 46.7%.
Embodiment 1-2
[0023] 13.0kg naphthalene, 0.52kg ionic liquid [Et 3 NH][HSO 4 ], 110kg of acetic acid and 13kg of co-solvent cyclohexane were added to the reactor, and the temperature was raised to 90°C under stirring, and 113.5kg of aqueous hydrogen peroxide solution with a mass percentage concentration of 30% was added dropwise within 1.5 to 2.5 hours. After 2 hours, the insoluble precipitate was filtered out, and the filtrate was extracted with cyclohexane, washed with water, dried, and then distilled under reduced pressure to obtain 9.42kg of 1,4-naphthoquinone with a yield of 58.7%.
Embodiment 1-3
[0025] 13kg naphthalene, 1.3kg ionic liquid [Et 3 N(CH 2 ) 4 SO 3 H][HSO 4 ], 65kg of acetic acid, 13kg of co-solvent (chlorobenzene:xylene = 1:3 mass ratio) were added to the reactor, the temperature was raised to 60°C under stirring, and 170kg of mass percentage was added dropwise within 1.5 to 2.5 hours. The concentration was 30% Aqueous hydrogen peroxide solution, dropwise, keep warm for 2.5 hours, filter out the insoluble precipitate, extract the filtrate with cyclohexane, wash with water, dry, then carry out vacuum distillation to obtain 6.32kg of 1,4-naphthoquinone with a yield of 39.4% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com