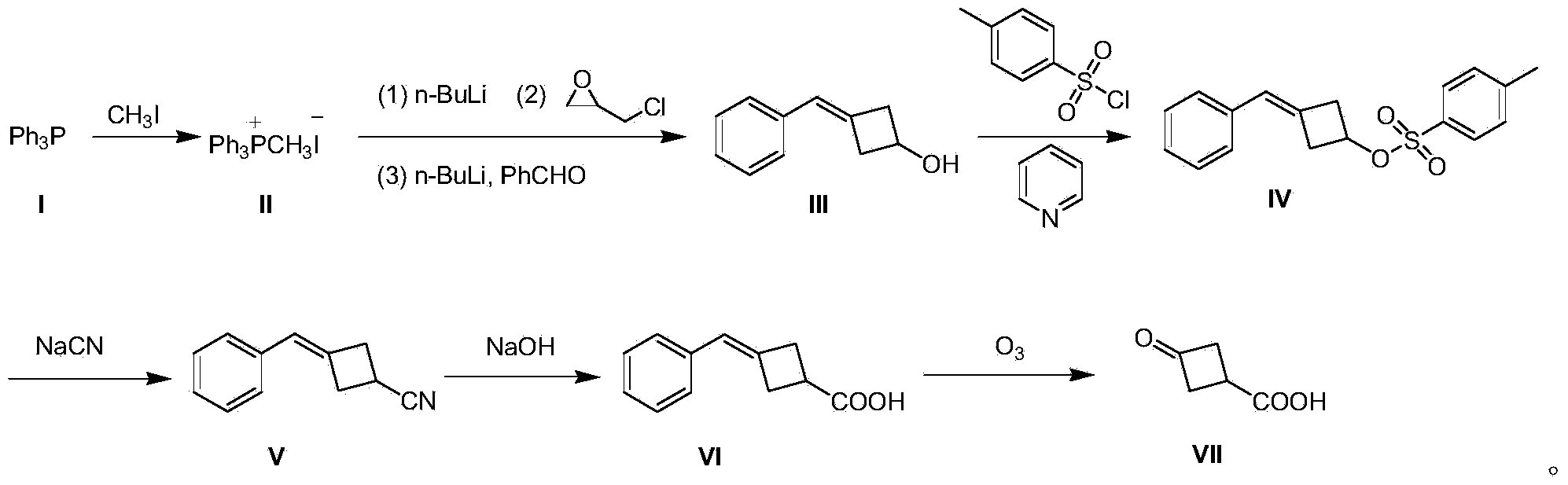
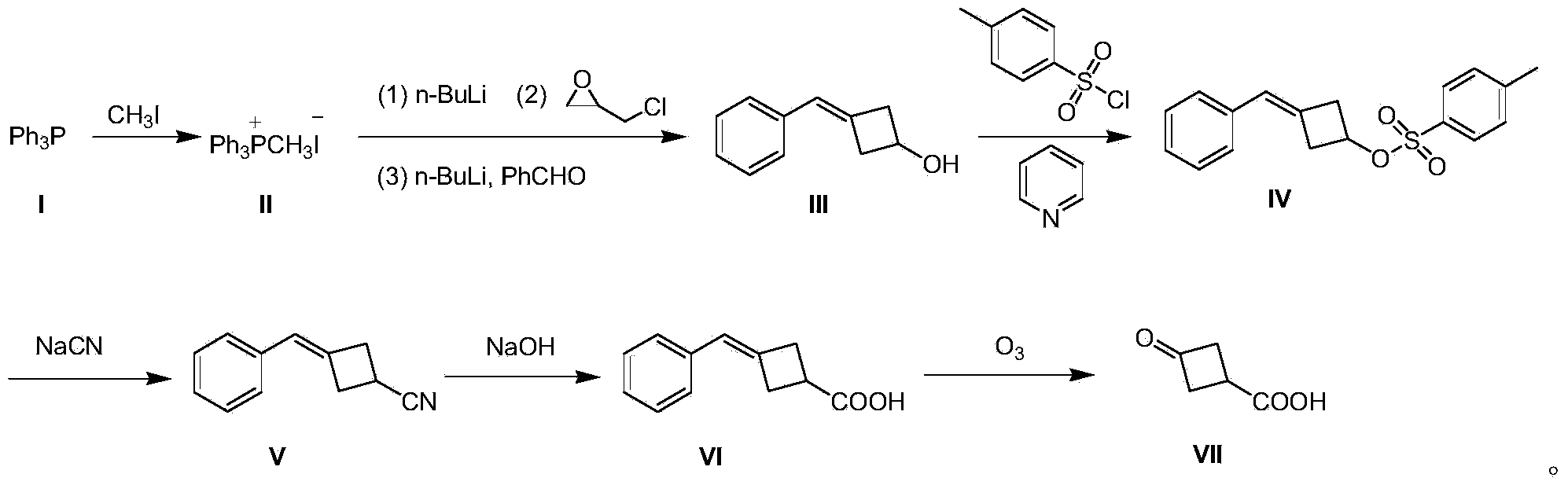
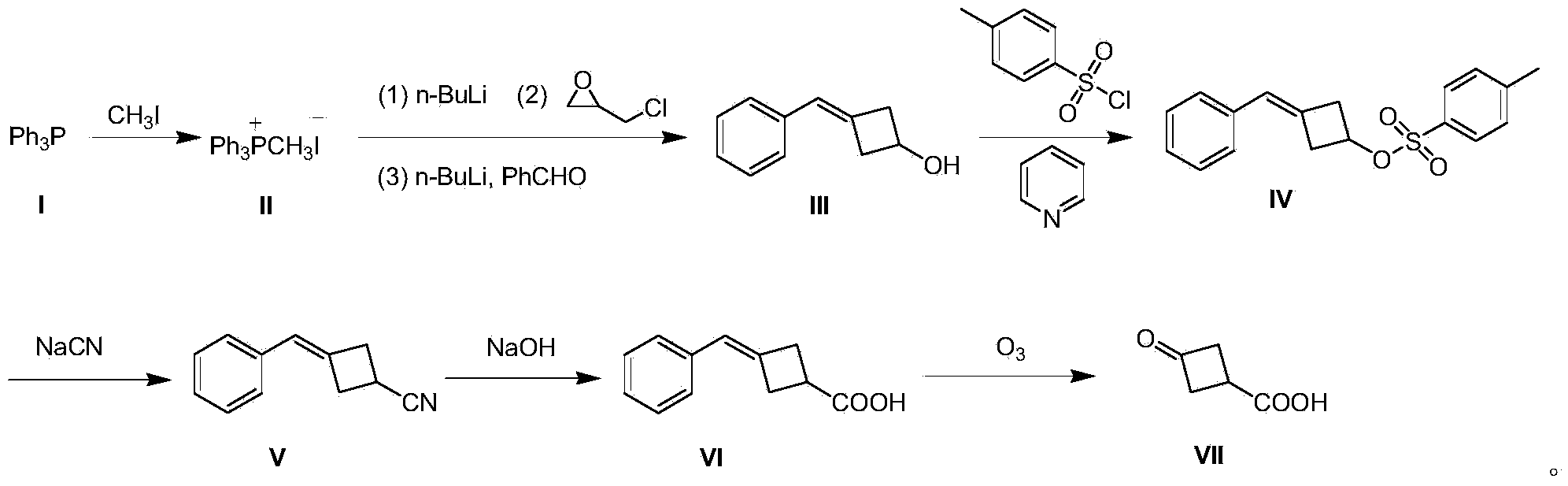
Preparation method of 3-oxocyclobutanecarboxylic acid
A technology of oxocyclobutane carboxylic acid and benzylidene cyclobutanol is applied in the field of preparation of 3-oxocyclobutane carboxylic acid, can solve the problem of high cost, achieve cost economy, technically simple and convenient operation effect used
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A preparation method of 3-oxocyclobutane carboxylic acid (VII), comprising the steps:
[0021] (1) Preparation of methyltriphenylphosphine iodide (II)
[0022] 500 g (2.03 mol) of triphenylphosphine (I) was dissolved in 3 L of tetrahydrofuran, 432 g (3.05 mol) of methyl iodide was added dropwise to the system, and the mixture was stirred at room temperature for 12 hours to produce a large amount of white solid, which was filtered to remove excess methyl iodide. And tetrahydrofuran to obtain solid methyl triphenylphosphine iodide (II) 850g, yield 91%;
[0023] (2) Preparation of 3-benzylidenecyclobutanol (III)
[0024] Methyltriphenylphosphine iodide (II) 40.4 g (0.1 mol) was dissolved in 800 mL of toluene, and the temperature was controlled at 0 °C, and 40 mL of n-butyllithium n-hexane solution, 2.5 M (0.1 mol), dripped in 15 minutes; warmed up to room temperature and stirred for 0.5 hours; then cooled to 0 °C, and added 9.2 g (0.1 mol) of epichlorohydrin dropwise at ...
Embodiment 2
[0036] A preparation method of 3-oxocyclobutane carboxylic acid (VII), comprising the steps:
[0037] (1) Preparation of methyltriphenylphosphine iodide (II)
[0038] Dissolve 500 g (2.03 mol) of triphenylphosphine (I) in 3 L of tetrahydrofuran, add 432 g (3.05 mol) of methyl iodide to the system dropwise, and stir at room temperature for 18 hours to produce a large amount of white solids, filter to remove excess methyl iodide And tetrahydrofuran to obtain solid methyl triphenylphosphine iodide (II) 857g, yield 92%;
[0039] (2) Preparation of 3-benzylidenecyclobutanol (III)
[0040]80.8 g (0.2 mol) of methyltriphenylphosphine iodide (II) was dissolved in 1500 mL of toluene, and at -3 °C under temperature control, 96 mL of n-butyllithium in n-hexane solution, 2.5 M ( 0.24mol), dripped in 30 minutes; warmed to room temperature and stirred for 1 hour; then cooled to -3°C, and at this temperature, 18.4g (0.2mol) of epichlorohydrin was added dropwise, and stirred at -3°C for 1 h...
Embodiment 3
[0050] A preparation method of 3-oxocyclobutane carboxylic acid (VII), comprising the steps:
[0051] (1) Preparation of methyltriphenylphosphine iodide (II)
[0052] Dissolve 1000 g (4.06 mol) of triphenylphosphine (I) in 5 L of tetrahydrofuran, add 864 g (6.1 mol) of methyl iodide to the system dropwise, and stir at room temperature for 24 hours to produce a large amount of white solids, filter to remove excess methyl iodide and tetrahydrofuran to obtain solid methyl triphenylphosphine iodide (II) 1775g, yield 95%;
[0053] (2) Preparation of 3-benzylidenecyclobutanol (III)
[0054] Methyltriphenylphosphine iodide (II) 40.4g (0.1mol) was dissolved in 800mL of toluene, and the temperature was controlled at -5°C, 40mL of n-butyllithium in n-hexane solution, 2.5M ( 0.1mol), dripped in 15 minutes; warmed up to room temperature and stirred for 1 hour; then cooled to -5°C, and at this temperature, 9.2g (0.1mol) of epichlorohydrin was added dropwise, stirred at -5°C for 1 hour, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com