Novel iridium complex and preparation method thereof as well as application thereof in organic electroluminescence device
An iridium complex and reaction technology, applied in the field of preparation of organic electroluminescent devices, can solve the problems of high energy consumption of devices, unfavorable device stability and lifespan, increase of device temperature, etc., and achieve the effect of good luminescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Synthesis of the main ligand L1-L4
[0038] Under oxygen-free nitrogen protection conditions, 25mmol2-bromopyridine, 30mmol2-(trifluorophenyl)boronic acid or 2-(tetrafluorophenyl)boronic acid, 0.35g (0.30mmol) tetrakis(triphenylphosphine) palladium catalyst And 16.56g (120mmol) of potassium carbonate, dissolved in 120mL tetrahydrofuran: water = 2:1 mixed solvent, heated to reflux at 80°C, and followed the reaction by thin layer chromatography. After the reaction was completed, it was cooled and extracted three times with 30 mL of dichloromethane (30 mL). Collect the organic phase, dry with anhydrous magnesium sulfate, and then use 300 mesh silica gel column chromatography, the eluent used is sherwood oil: ethyl acetate=10:1 mixed solvent, obtain white solid L1-L4, yield 82- 90%. The characterization data of the obtained compound are as follows:
[0039] main ligand L1
[0040] Yield: 82%
[0041] 1 HNMR (500MHz, acetone-d 6 ,ppm)δ8.69(d,J=4.7Hz,1H),8.02...
Embodiment 2
[0059] Synthesis of Example Two Auxiliary Ligand AL and Its Potassium Salt KAL
[0060] Under anhydrous and oxygen-free conditions, 20mmol of phosphorus chloride with different substituents was dissolved in 30mL of anhydrous toluene, heated to reflux, and 1.77g (11mmol) of hexamethyldisilamine (HMDS) was slowly added dropwise. Continue to react under reflux for 6h. After the reaction is over, cool down and remove the reaction by-product trimethylchlorosilane and solvent by rotary evaporation. Then it was dissolved in 4mL THF, and the mixed solution of 2mL 30% hydrogen peroxide and 5mL THF was slowly added dropwise to the reaction liquid, and reacted for 2h after the dropwise completion. After the end, the liquid was poured into 50 mL of ether solution, and a large amount of white precipitates were formed. The precipitates were washed with water and dried in vacuum to obtain the product AL.
[0061] At room temperature, slowly drop 10mL of 2% methanolic potassium hydroxide sol...
Embodiment 3
[0092] The synthesis of embodiment three iridium complexes
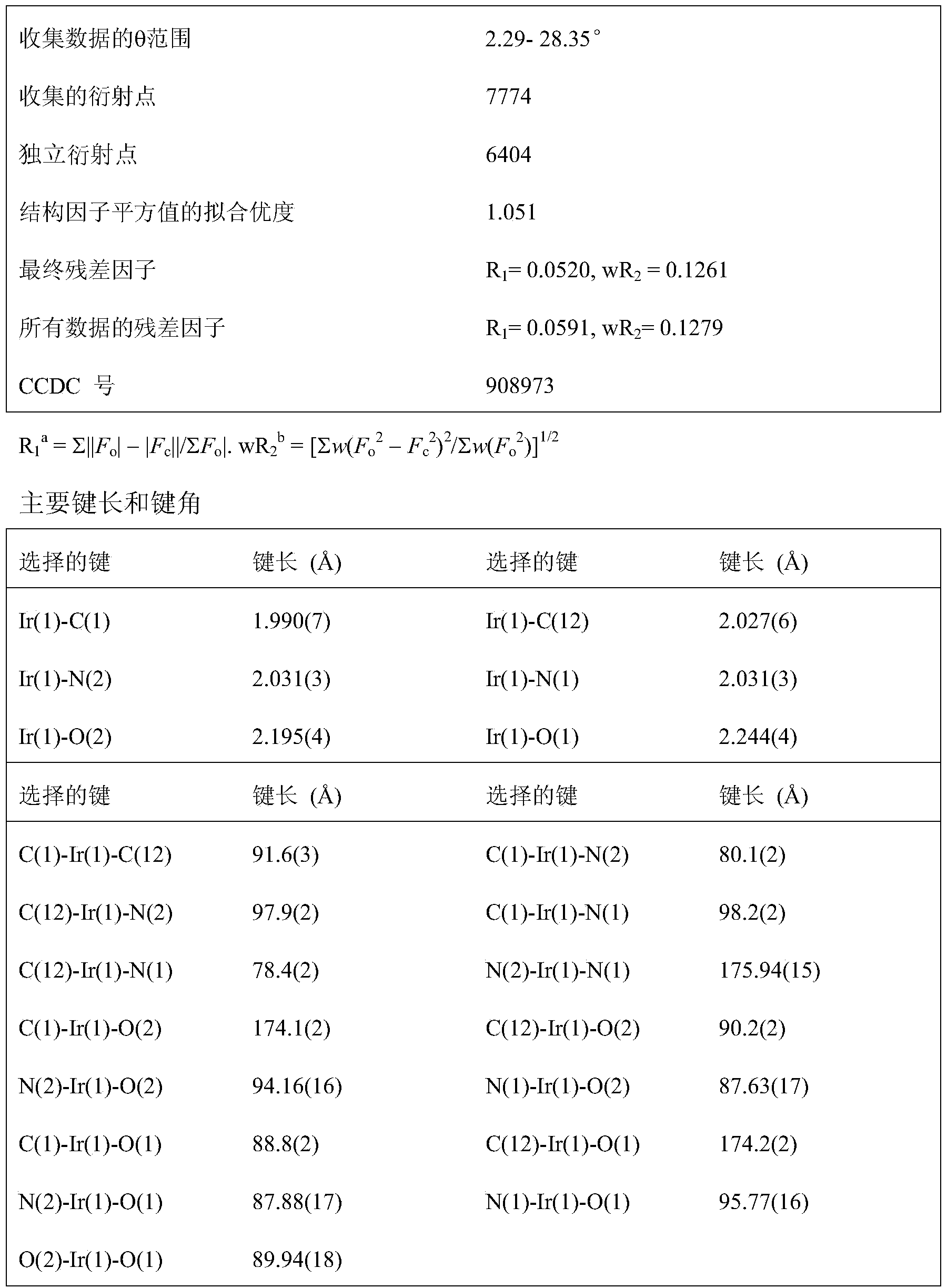
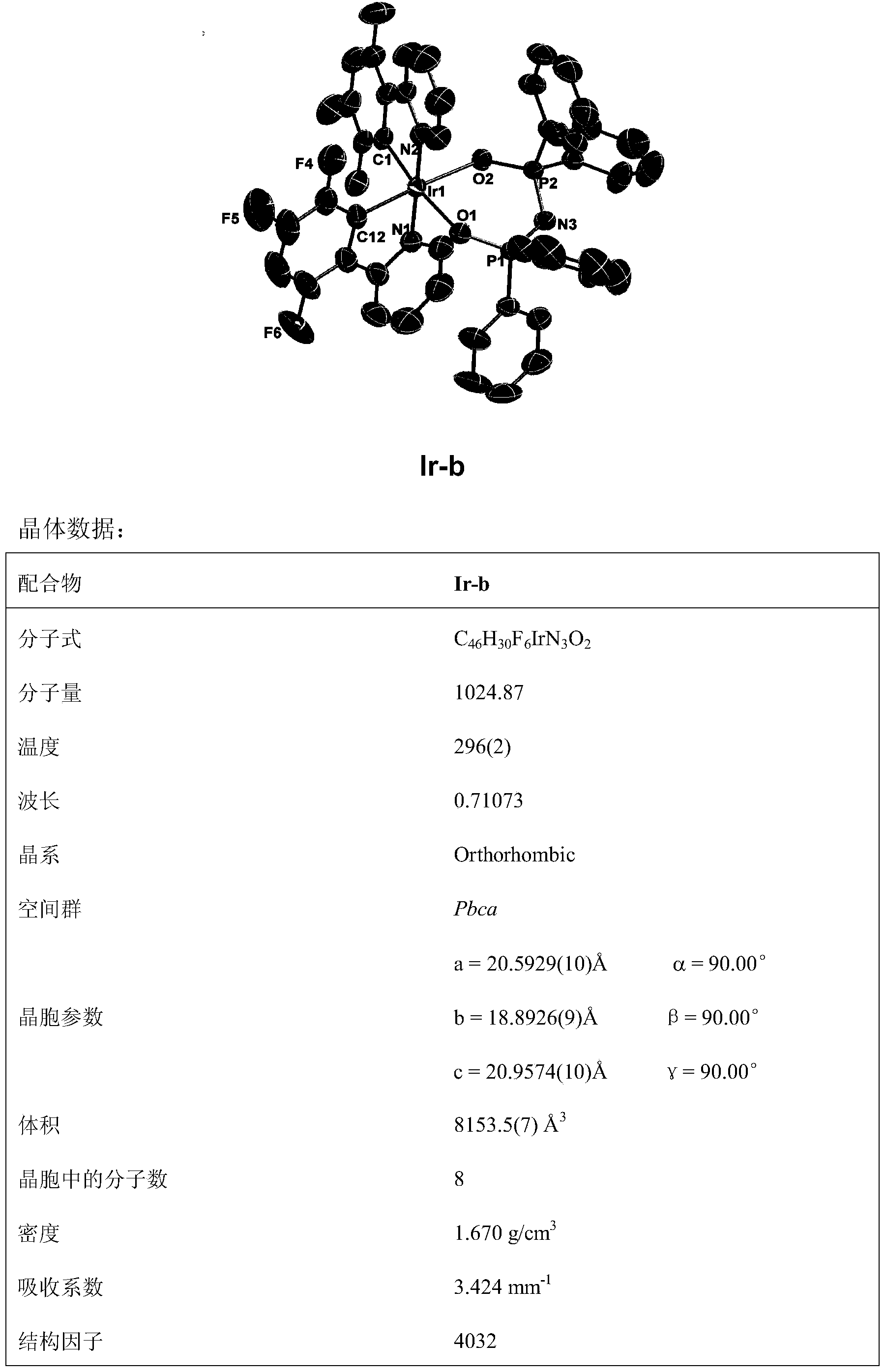
[0093] The reaction is carried out under anhydrous and anaerobic operation, 0.20mmol of [(F-ppy) 2 Ir(μ-Cl)] 2 and 2.5 times the equivalent of KAL (0.5mmol) were dissolved in 10mL of ethylene glycol monoethyl ether solution, and reacted at 120°C for 24h. After the reaction, a small amount of yellow precipitate was formed, and the solvent was distilled off under reduced pressure. Silica gel column chromatography, the eluent used is a mixed solvent of petroleum ether: ethyl acetate with a volume ratio of 10:1-7:1. The product was precipitated by adding petroleum ether to obtain a yellow solid, which was dried in vacuum and purified by sublimation to obtain an iridium complex, melting point: >310°C.
[0094] The characterization data of the obtained compound are as follows:
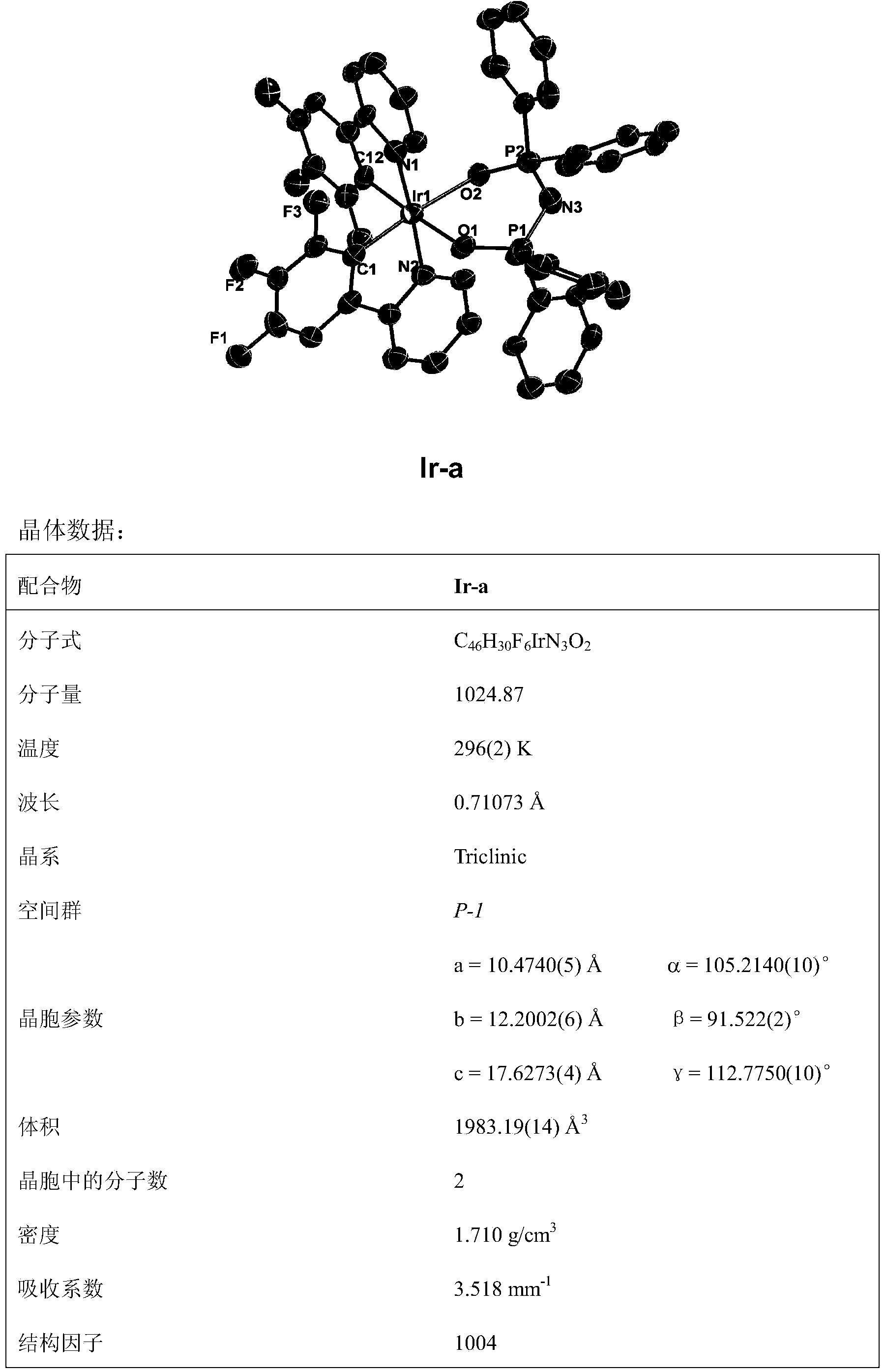
[0095] Complex Ir-a
[0096] Yield: 28%
[0097] 1 HNMR (500MHz, CDCl 3 ,ppm)δ9.03(d,J=5.6Hz,2H),7.77(dd,J=12.3,7.0Hz,4H),7.50(d,J=8.1Hz,14...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com