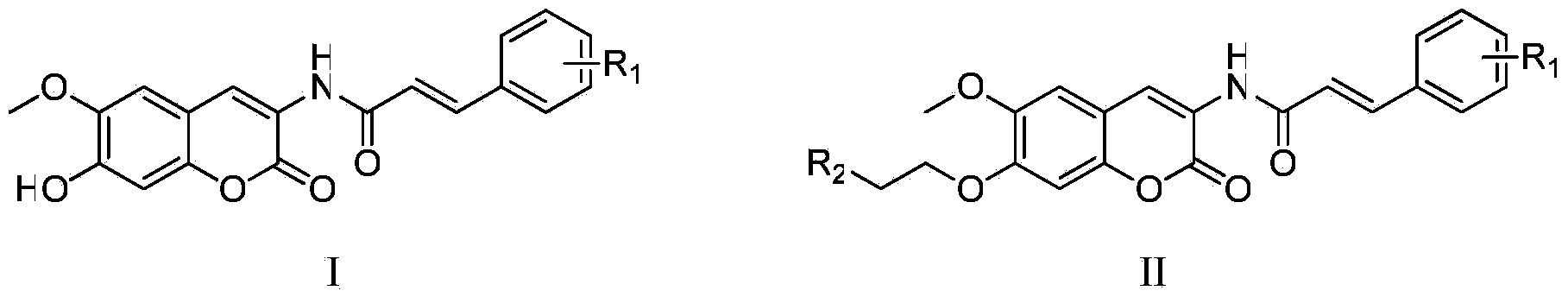
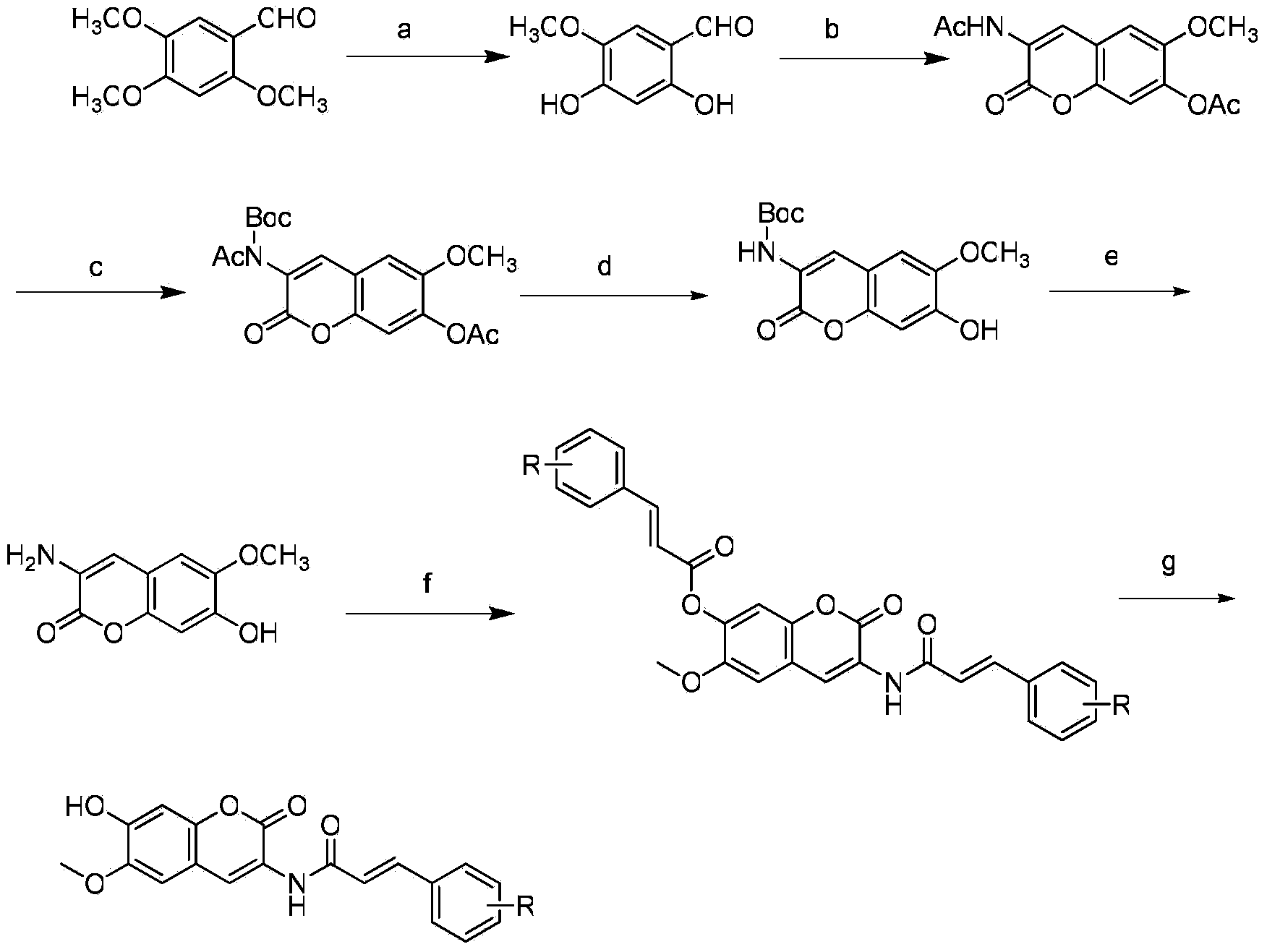
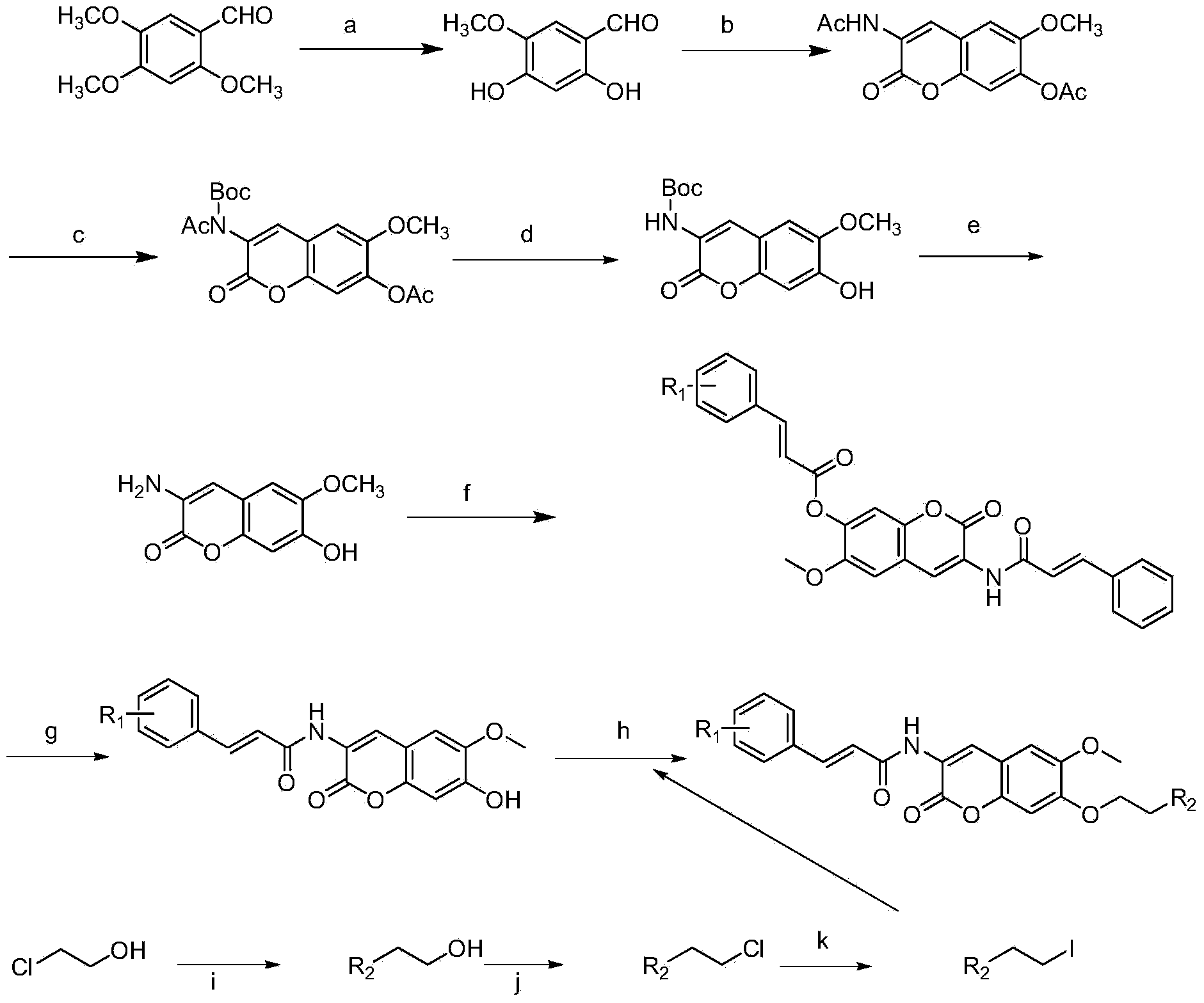
Scopoletin derivatives with anti-tumor activity, and preparation method and application thereof
A tumor and drug technology, applied in the field of scopolamine derivatives, can solve the problems of unstable metabolism, high effective concentration, and short action time in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of 2,4-dihydroxy-5-methoxybenzaldehyde:
[0049] Add aluminum trichloride (80g, 0.60mol) and anhydrous dichloromethane (400mL) into a 1000mL three-necked flask, then add 1gCTAB, and stir. 2,4,5-Trimethoxybenzaldehyde (20g, 0.10mol) was dissolved in anhydrous dichloromethane (100mL), added dropwise to the reaction flask, and heated to reflux for 4h. Pour the reactant into 500 g of crushed ice to which 100 mL of concentrated hydrochloric acid has been added, stir for 0.5 h, separate the organic layer, wash the organic layer three times with saturated aqueous sodium chloride solution, dry over anhydrous sodium sulfate, evaporate the solvent by rotary evaporation, and recrystallize from toluene , to obtain a light yellow solid (15.50g, 90.42%), mp.145℃.
[0050] 1 H NMR (300MHz, DMSO-d 6 ):δ3.71(3H,s,OCH 3 ), 3.94 (1H, s, OH), 6.50 (1H, s, phenyl1-H), 6.69 (1H, s, phenyl1-H), 9.80 (1H, s, OH), 10.70 (1H, s, CHO) .ESI-MS(m / z):168[M-H] - .
[0051] Preparat...
Embodiment 2
[0063] 3-(4-Chlorophenyl)-N-(6-methoxy-7-hydroxy-2H-1-benzopyran-2-one-3-yl)acrylamide (Ⅰ 2 )
[0064] Referring to the preparation method of Example 1, using p-chlorocinnamic acid and 6-methoxy-7-hydroxy-3-aminocoumarin as raw materials, recrystallized from ethanol to obtain about 120mg, yellow-white powder, yield 81.4%, mp >250°C.
[0065] ESI-MS(m / z):366[M-H]-.
[0066] IR(KBr,cm -1 ):3334(OH);3120(C=C);1708,1677(C=O).
[0067] 1 H NMR (DMSO-d 6 , 300MHz) δ: 3.83 (3H, m, OCH 3 ), 6.81 (1H, s, H8), 7.28 (1H, d, J=13.8Hz, CH), 7.35 (2H, d, J=8.1Hz, Ar-H), 7.51 (1H, s, H5), 7.53(2H,d,J=8.1Hz,Ar-H),7.64(1H,d,J=13.8Hz,CH),8.73(1H,s,H4),9.76(1H,s,NH),10.10( 1H,s,OH).
Embodiment 3
[0069] 3-(4-fluorophenyl)-N-(6-methoxy-7-hydroxy-2H-1-benzopyran-2-one-3-yl)acrylamide (Ⅰ 3 )
[0070] Referring to the preparation method of Example 1, using p-fluorocinnamic acid and 6-methoxy-7-hydroxy-3-aminocoumarin as raw materials, recrystallized from ethanol to obtain about 125mg, yellow powder, yield 75.2%, mp> 250°C.
[0071] ESI-MS(m / z):354[M-H] - .
[0072] IR(KBr,cm -1 ):3331(OH);3144(C=C);1710,1673(C=O).
[0073] 1 H NMR (DMSO-d 6 , 300MHz) δ: 3.83 (3H, m, OCH 3 ), 6.81 (1H, s, H8), 7.23 (1H, d, J=13.8Hz, CH), 7.32 (2H, d, J=8.1Hz, Ar-H), 7.49 (1H, s, H5), 7.54(2H,d,J=8.1Hz,Ar-H),7.67(1H,d,J=13.8Hz,CH),8.86(1H,s,H4),9.84(1H,s,NH),10.17( 1H,s,OH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com