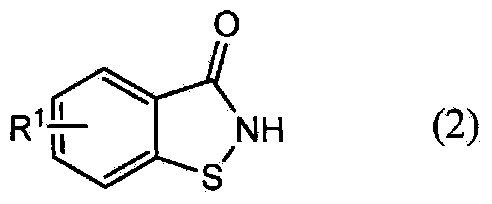
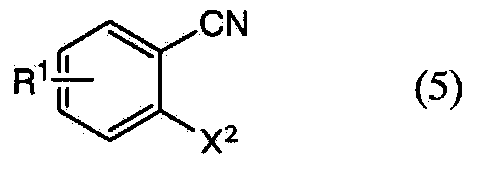
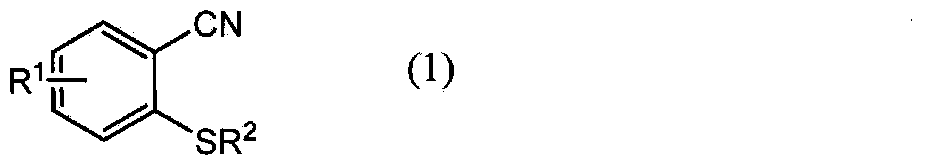Method for producing 1,2-enzisothiazol-3-one compound
A technology of benzisothiazole and ketone compounds, applied in the direction of organic chemical methods, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problems of increasing waste water and undesired needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] (i) Synthesis of methyl mercaptan
[0135] A 20% aqueous solution (280 g) of sodium hydrosulfide (1.00 mol), chlorobenzene (200 g), and a 50% by weight aqueous solution (3.2 g) of tetra-n-butylammonium bromide were placed under a nitrogen atmosphere equipped with a stirrer, In a 500mL four-necked flask with a thermometer, a condenser and a gas blow tube. The mixture was stirred at 30°C.
[0136] 2-(Methylthio)benzonitrile (298.0 g, 2.0 moles), monochlorobenzene (460.0 g) and 35% by weight hydrochloric acid (67.0 g, hydrochloric acid: 0.6 moles, water: 2.4 moles) were placed in a stirrer equipped with , a thermometer and a condenser in a 3L four-necked flask. Sulfonyl chloride (270.0 g, 2.0 mol) was added thereto while stirring at 5°C to 15°C. The mixture was further heated to 65-70°C and allowed to react for 1 hour. Gas generated during the reaction was passed through 15% aqueous sodium hydroxide to remove hydrogen chloride. The obtained methyl chloride gas (50.5...
Embodiment 2
[0145] (i) Synthesis of ethanethiol
[0146] Under a nitrogen atmosphere, a 20% aqueous solution (280 g) of sodium hydrosulfide (1.00 mol), chlorobenzene (100 g) and a 50% by weight aqueous solution (3.2 g) of tetra-n-butylammonium bromide were placed in a device equipped with a stirrer, a thermometer, , condenser and blow tube in a 500mL four-necked flask. The mixture was stirred at 30°C.
[0147] 2-(Ethylthio)benzonitrile (326.0 g, 2.0 moles), monochlorobenzene (460.0 g) and 35% by weight hydrochloric acid (67.0 g, hydrochloric acid: 0.6 moles, water: 2.4 moles) were placed in a stirrer equipped with , a thermometer and a condenser in a 3L four-necked flask. While stirring, chlorine gas (156.0 g, 2.2 mol) was blown thereinto at 45°C˜50°C over 2 hours, and the resulting mixture was further heated to 65°C˜70°C and then allowed to react for 1 hour. Gas generated during the reaction was passed through 15% aqueous sodium hydroxide to remove hydrogen chloride. The resulting ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com