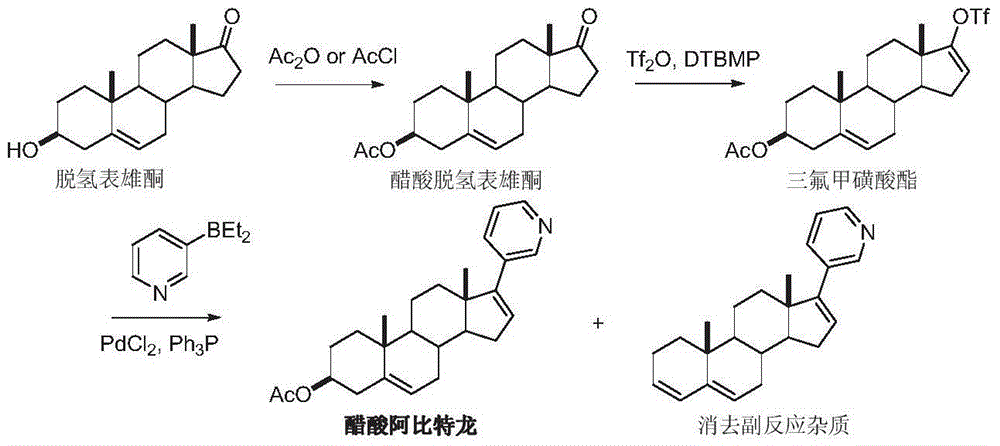
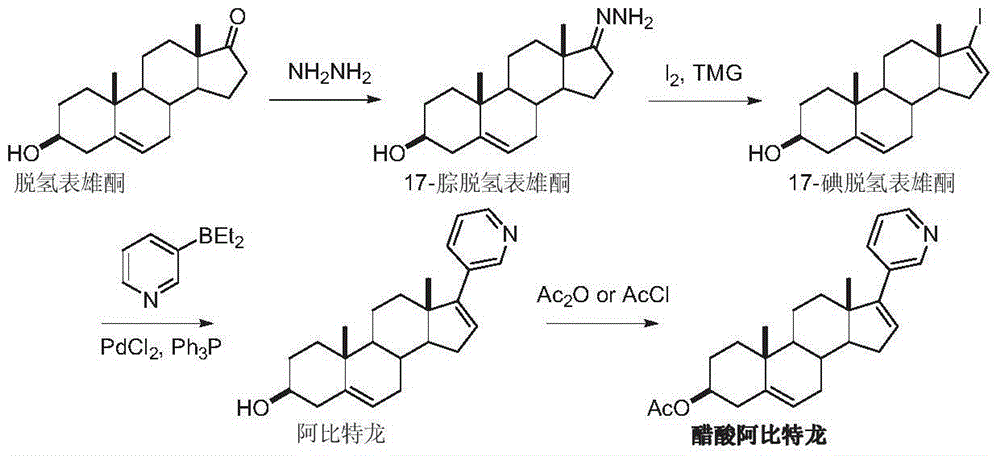
The preparation method of Abiraterone
An abiraterone, drying technology, applied in the direction of steroids, organic chemistry, etc., can solve the problems of long coupling reaction time, low yield, difficult purification, etc., to promote economic and technological development, economical environmental protection, reliable raw materials easy-to-get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add dehydroepiandrosterone (II) (7.2g, 25mmol) and dichloromethane 100mL in the dry reaction bottle, add chloromethyl methyl ether (8.0g, 100mmol) and diisopropylethylamine ( 13.6 g, 105 mmol), the temperature was raised to reflux for 12 hours, and TLC detected the end of the reaction. After cooling, the reaction solution was poured into ice water, the organic phase was separated, the aqueous phase was extracted twice with dichloromethane, the organic phases were combined, and dried over anhydrous magnesium sulfate. Dichloromethane was recovered under reduced pressure, and the residue was recrystallized from n-hexane to obtain 6.95 g of 3β-methoxymethyl ether dehydroepiandrosterone (II), with a yield of 83.7%.
Embodiment 2
[0038] Add dehydroepiandrosterone (II) (2.88g, 10mmol) and 50mL of dichloromethane in the dry reaction flask, add chloromethyl methyl ether (2.0g, 25mmol) and Na-Y zeolite (0.72g , 25% w / w), the temperature was raised to reflux for 7 hours, and TLC detected that the reaction was complete. After cooling, the solid was removed by filtration, and the organic phase was washed successively with 10% sodium bicarbonate solution, water and brine, and dried over anhydrous sodium sulfate. Dichloromethane was recovered under reduced pressure, and the residue was recrystallized from n-hexane to obtain 3.0 g of 3β-methoxymethyl ether dehydroepiandrosterone (II), with a yield of 90.4%.
Embodiment 3
[0040]Under the protection of nitrogen, add 3-pyridylmagnesium bromide in 2-methyltetrahydrofuran solution (1.0M, 12mL, 12mmol) into the dry reaction flask, cool down to 0-5°C, add 3β-methoxymethyl ether dropwise Dehydroepiandrosterone (II) (3.2 g, 10 mmol) in 2-methyltetrahydrofuran (50 mL) was reacted at a temperature below 20° C. for 1 hour. Add 50 mL of toluene and cool down to 5°C. Add 25% hydrochloric acid, stir for 30 minutes, let stand, separate the organic phase, wash with water twice, and dry over anhydrous magnesium sulfate. Toluene was recovered under reduced pressure, and the residue was recrystallized from ethyl acetate and n-hexane to obtain 3.75 g of 17-(3-pyridine)-17-hydroxy-androst-5-ene-3β-methoxymethyl ether (III) , yield 91.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com