Multiple target point furanone-quinolinone compounds and preparation method and usage of multiple target point furanone-quinolinone compounds
A technology of furanone and quinolinone, which is applied in the application field of preparing antibacterial drugs, to achieve the effect of good inhibition and killing effect and high antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
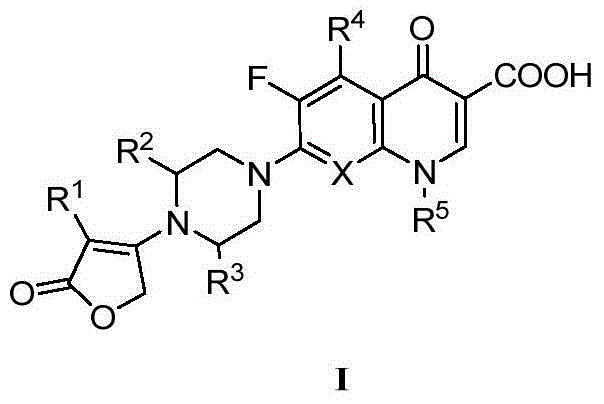
[0023] Example 1: 3-(2-chlorophenyl)-4-(4-(1-cyclopropyl-3-carboxy-6-fluoro-4-quinolinone-7-yl)piperazin-1-yl )-2(5H)-furanone (7)
[0024] Step 1: Dissolve 1.9g of sodium 2-chlorophenylacetate in 20mL of DMSO, add 1.8mL of ethyl bromoacetate at room temperature, heat up to 35°C and react for 8 hours. After the reaction is complete, dilute with ethyl acetate, wash with water, and wash the organic layer with Wash with saturated brine until neutral, dry, concentrate, use silica gel column chromatography, eluent is petroleum ether-AcOEt, the volume ratio of petroleum ether and AcOEt is 7:1, obtain 2.3g oily 2-(2-chlorophenylacetyl Oxy) ethyl acetate;
[0025] Step 2: Dissolve 2.0 g of ethyl 2-(2-chlorophenylacetoxy)acetate in a constant-pressure funnel containing 10 mL of anhydrous THF, add 0.21 g of NaH into a flask containing 5 mL of anhydrous THF, A THF solution of ethyl 2-(2-chlorophenylacetoxy)acetate was added slowly with stirring. Stir the reaction at room temperature f...
Embodiment 2
[0037] Embodiment 2: the antibacterial activity of compound
[0038]Bacteria were suspended in MH medium at a concentration of approximately 10 5 cfu mL -1 , add the bacterial solution to a 96-well plate (100 μL of bacterial solution per well), use the culture medium as a blank control, use DMSO instead of a test substance as a negative control, use penicillin G as a positive control for Gram-positive bacteria, and use penicillin G as a positive control for Gram-positive bacteria. Kanamycin was used as a positive control for Shi-negative bacteria, and ketoconazole was used as a positive control for fungi. Dissolve the test substance in DMSO to prepare 1600, 800, 400, 200, 100, 50 μg·mL -1 solution (for MIC 50 Less than 5μg·mL -1 Yes, when carrying out one-step experiment, the prepared concentration gradient is 100, 50, 25, 12.5, 6.25 μg·mL -1 ), was added to a 96-well plate at an amount of 11 μL per well, and four parallel experiments were performed for each concentration...
Embodiment 3
[0041] Example 3: Extraction of TyrRS and determination of the activity of compounds on TyrRS
[0042] TyrRS from Staphylococcus aureus was expressed in E. coli and purified by Sephadex chromatography. The activity of TyrRS was determined by aminoacylation reaction. The enzyme reaction mixture consists of the following components: 100mM TrisHCl pH7.9, 50mM KCl, 16mM MgCl 2 , 5mM ATP, 3mM dithiothreitol, 4mg / mL Escherichia coli MRE600tRNA and 10μM[ 3 H] Tyrosine (activity 1.48-2.22TBq / mmol). Mix and incubate TyrRS (0.2nM) and different concentrations of test substances at room temperature for 10 minutes, then add an equal amount of the above enzyme reaction mixture preheated to 37°C, and after co-incubating for 5 minutes, add an equal volume of 7% glacial trichloro Acetic acid solution was used to terminate the reaction, filtered through a 96-well Millipore filter plate, and the filtrate was detected by a scintillation counter, and each sample was repeated 4 times. The one ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com