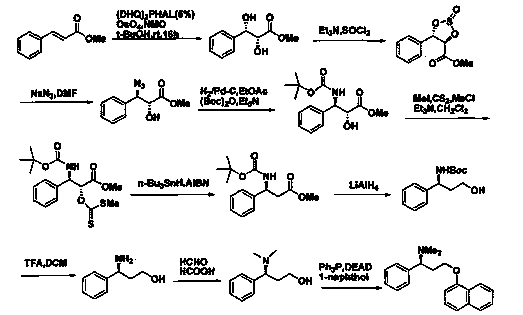
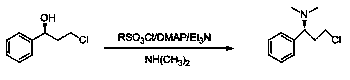(S)-3-chloro-N, N-dimethyl-1-phenyl-1-propylamine and method for preparing dapoxetine by using same as intermediate
A technology of dapoxetine hydrochloride and dimethyl, applied in the field of treatment of male premature ejaculation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0040] Below in conjunction with preferred embodiment, the present invention is further described, but the present invention is by no means limited to following embodiment.
[0041] The (R)-3-chloro-1-phenylpropanol used in the present invention was purchased from ***, and other raw materials were purchased from Sinopharm Group.
[0042] 1. Preparation of (S)-3-chloro-N,N-dimethyl-1-phenyl-1-propanamine (3)
[0043] (R)-3-chloro-1-phenylpropanol 3.07g (18.00mmol), dimethylaminopyridine 0.21g (1.75mmol), triethylamine 3.64g (36.00mmol), add 12ml tetrahydrofuran, stir to dissolve, in At 0°C, add dropwise a mixture of 4.12g (36.00mmol) of methanesulfonyl chloride and 3ml of tetrahydrofuran, the dropwise addition is completed within 10 minutes, and the reaction is 6hr; Remove the solvent under pressure, add an appropriate amount of water, adjust the pH to 12-13 with 20% sodium hydroxide solution, add ethyl acetate to extract 3 times, combine the organic layers, wash with water, w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com