Application of milrinone in preparation of medicine for treating diabetic foot ulcer
A technology for diabetic foot ulcers and milrinone, which is applied in the fields of biotechnology and medicine, can solve the problems of few short-term follow-up studies of patients with DFD or DFU, ulcer recurrence, and the risk of re-amputation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The selection of embodiment 1 research object
[0024] Inclusion criteria: patients with type 1 or type 2 diabetes, meeting the WHO definition of diabetic foot disease, that is, diabetic patients with lower extremity infection, ulceration and / or destruction of deep tissues due to neuropathy and various degrees of peripheral vascular disease ; DFU patients with Wagner grade 1-5; meet the Framingham diagnostic criteria for heart failure, and NYHA grade Ⅱ-Ⅲ; blood pressure ≥ 90 / 60mmHg.
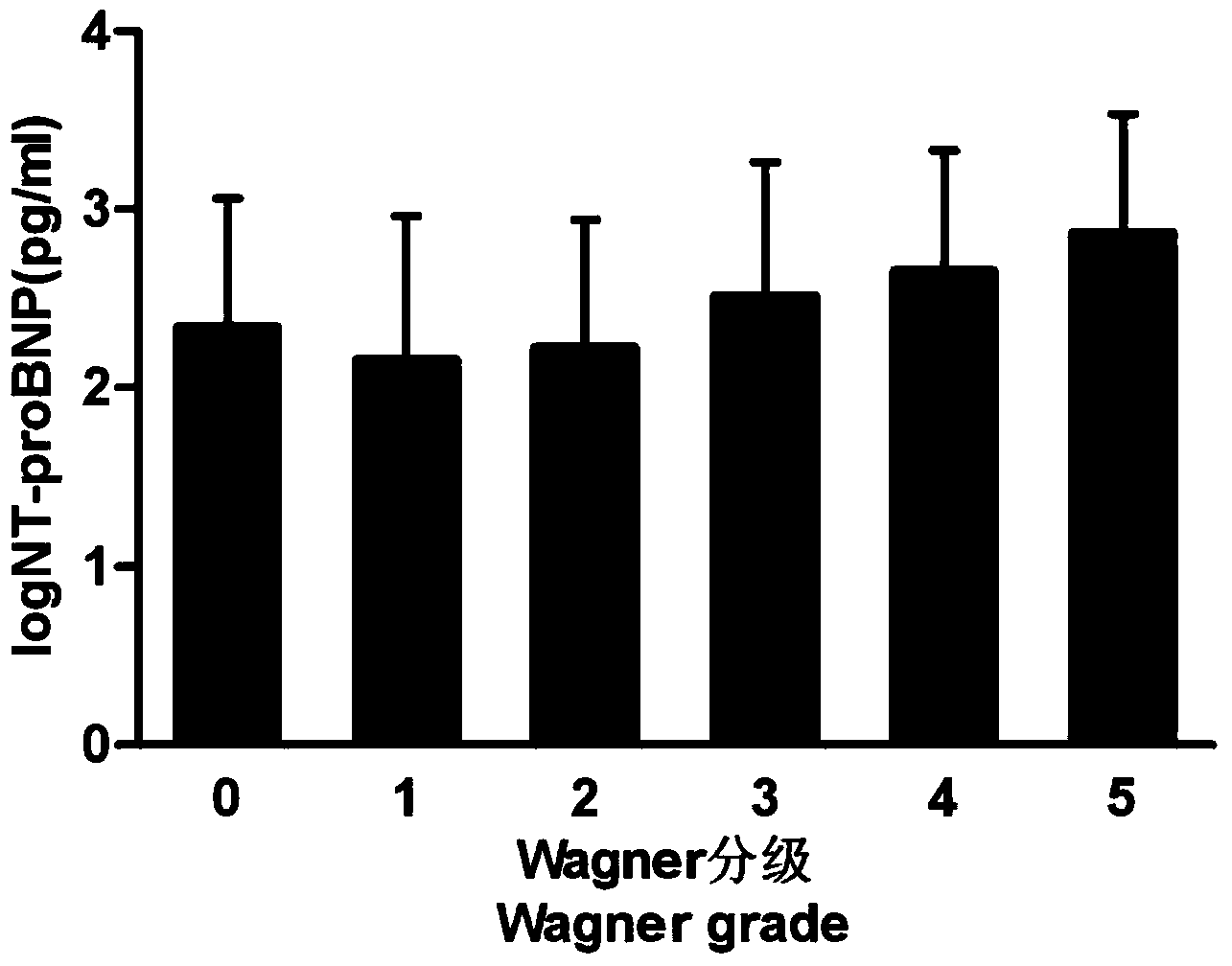
[0025] The severity of DFD is graded using the Wagner grading method, and the specific grades are as follows: grade 0, skin intact (before ulceration), with peripheral vascular disease and high-risk signs of the foot; grade 1, skin surface ulcers, subcutaneous Within the fat layer; Grade 2, ulcer depth reaches tendons or ligaments; Grade 3, osteomyelitis or extensive infection of deep tissues; Grade 4, gangrene of the toes or front of the foot; Grade 5, gangrene of the entire or involving ...
Embodiment 2
[0036] Embodiment 2 Administration
[0037] Using a randomized, controlled, single-blind method, the DFU patients included in the study were randomly divided into an intensive treatment group and a conventional treatment group according to different treatment schemes. A total of 142 patients with DFU combined with heart failure were included in the study, including 67 cases in the intensive treatment group, 65 cases were completed, 1 case was lost to follow-up, and 1 case was withdrawn from the study due to drug intolerance; 75 cases were in the conventional treatment group, 72 cases were completed, and 3 cases were lost to follow-up. example. There was no significant difference in the number of cases lost to follow-up between the two groups (P>0.05).
[0038] Conventional treatment group: conventional treatment, that is, according to the guidelines for the diagnosis and treatment of heart failure proposed by AHA, losartan potassium (50 mg, orally, once a day) was given to di...
Embodiment 3
[0044] There are 3 time nodes in the study: baseline period, 2 weeks and 3 months of treatment.
[0045] Statistical methods: Statistical processing was carried out using the Microsoft Windows version of SAS9.13 statistical software package. The measurement data satisfying the normal distribution is expressed by x±s, the comparison between two groups is performed by t test, and the comparison between multiple groups is performed by analysis of variance, while the measurement data of non-normal distribution is expressed by M(P25, P75), and logarithmic transformation is used Then compare between groups. The count data are expressed as ratios, and the four tables and the x of R×C are used 2 The test was compared with Fisher's exact test. All tests were two-sided, and P<0.05 was defined as statistically significant.
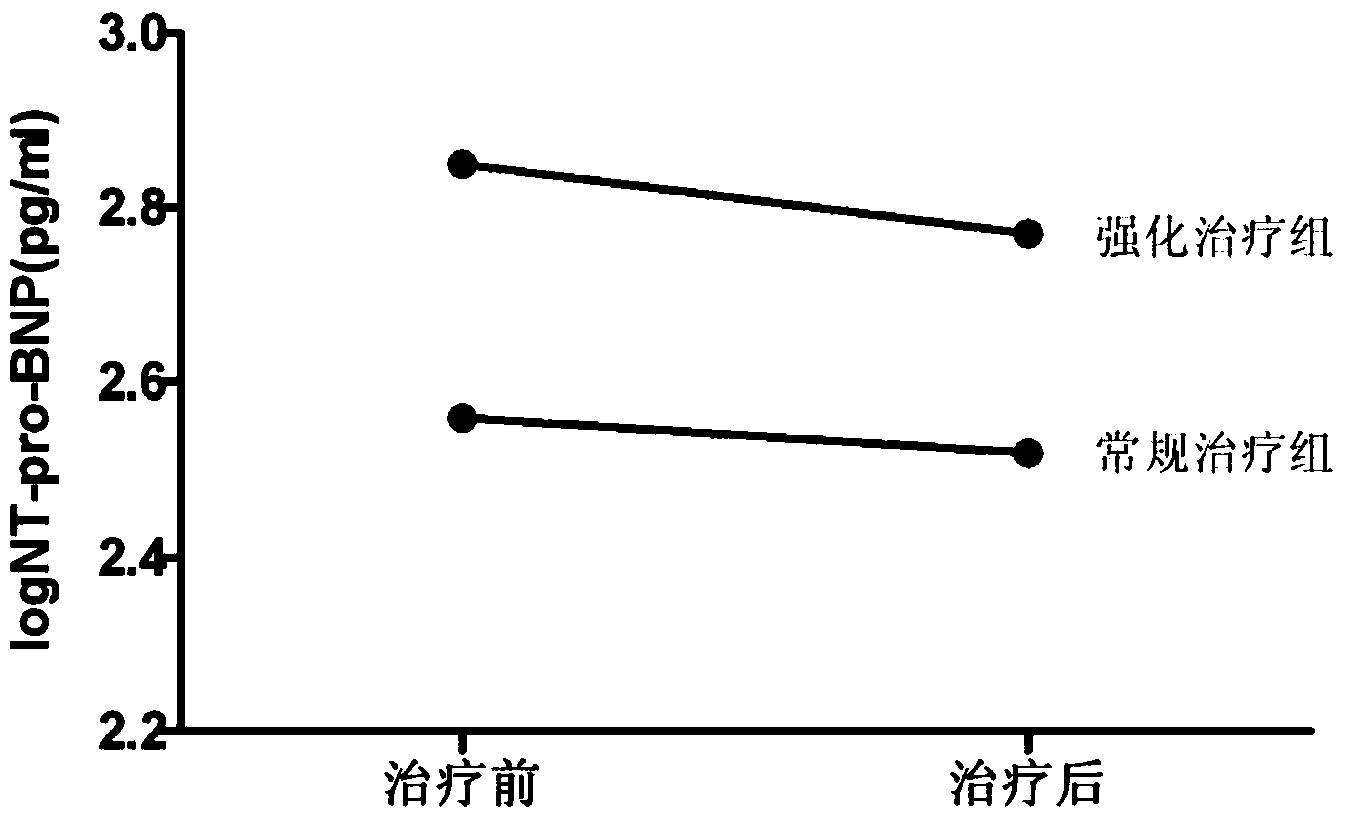
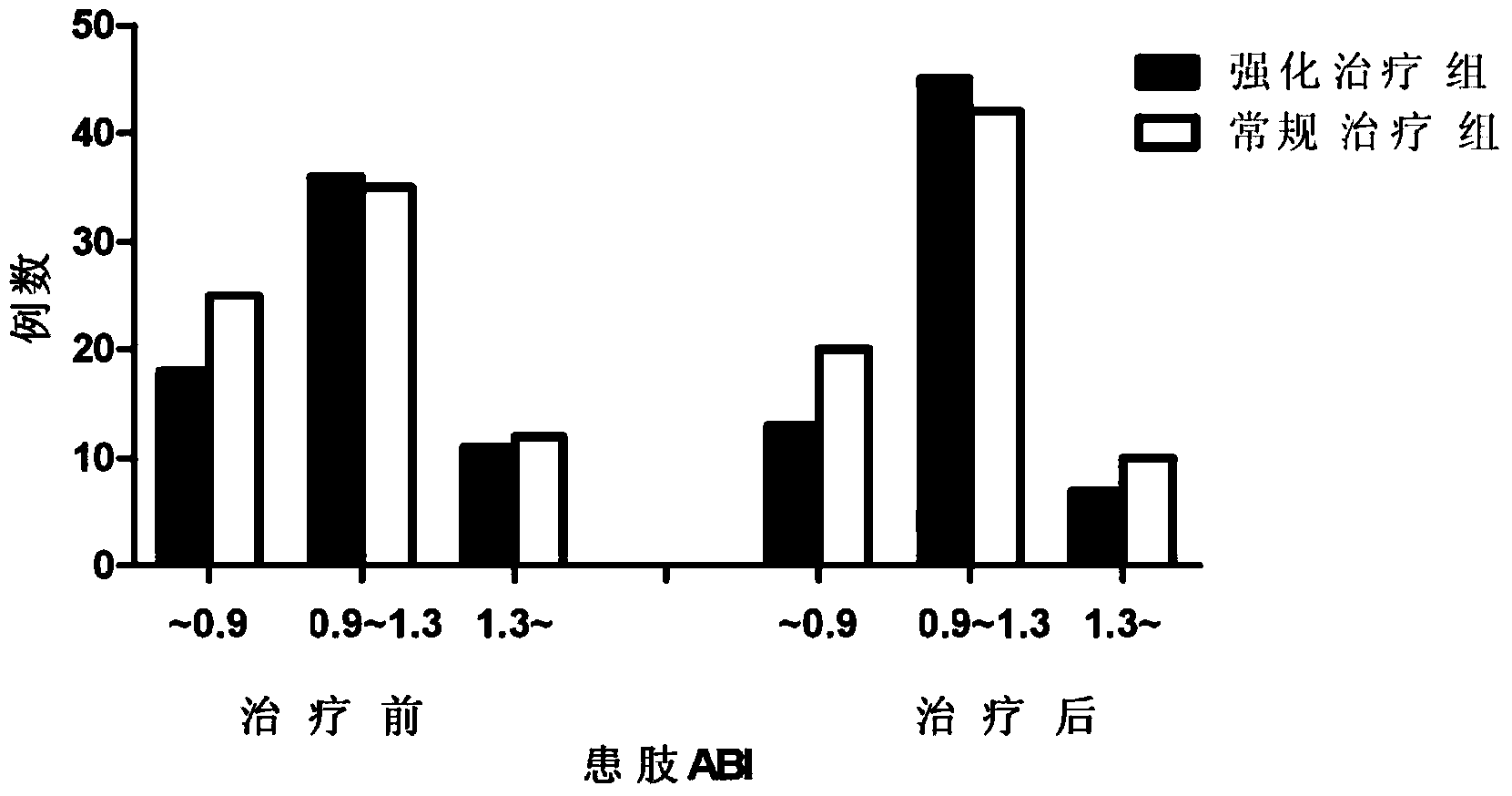
[0046] By monitoring the changes of ankle-brachial index (ABI) in patients with foot ulcers before and after treatment, it was observed whether the intensive trea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com