Humectant 1-o-hydroxyethyl-d-mannopyranose for tobacco and preparation method thereof
A technology of mannose and humectant for tobacco, which is applied in the preparation of sugar derivatives, chemical instruments and methods, tobacco, etc., and can solve the problems of weak binding ability of humectant, no moisture-proof effect, and affecting taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
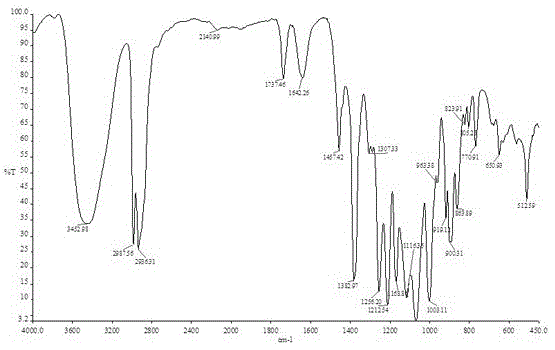
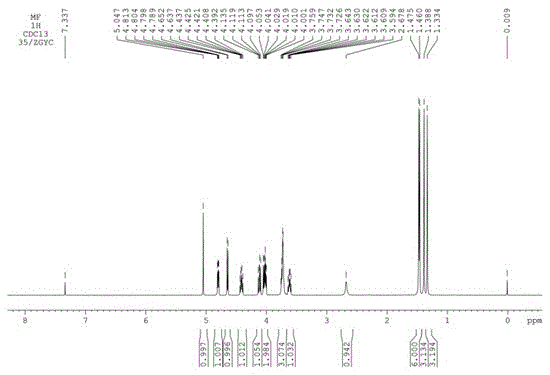
Image
Examples
Embodiment 1
[0030] The preparation method of 1-O-hydroxyethyl-D-mannopyranose, it comprises the steps:
[0031] (1) Preparation of 1-O-hydroxyethyl-2,3:5,6-di-O-isopropylidene-α-D-mannofuranose (Ⅱ)
[0032] Weigh 6.0 g of 1-O-carboxymethyl-2,3:5,6-di-O-isopropylidene-α-D-mannofuranosyl tert-butyl ester (Ⅰ) into a 250 mL three-necked flask, add 51 mL THF, stirred by magnetic force to dissolve, then protected by nitrogen, cooled in ice-water bath to below 10°C (preferably between 0-10°C), then slowly added LiAlH40.027mol, raised the temperature and reacted at 10°C 3h, TLC monitored the entire reaction process (V petroleum ether: V ethyl acetate = 3:2), then slowly dropwise added 18mL ethyl acetate aqueous solution (V ethyl acetate: V water = 1:1) to end the reaction, filtered the precipitate, Concentration under reduced pressure gave 3.469 g of a colorless transparent viscous liquid (II), with a yield of 71.13%.
[0033] (2) Preparation of 1-O-hydroxyethyl-D-mannopyranose (Ⅲ)
[0034] Ad...
Embodiment 2
[0036] The preparation method of 1-O-hydroxyethyl-D-mannopyranose, it comprises the steps:
[0037] (1) Preparation of 1-O-hydroxyethyl-2,3:5,6-di-O-isopropylidene-α-D-mannofuranose (Ⅱ)
[0038] Weigh 6.0 g of 1-O-carboxymethyl-2,3:5,6-di-O-isopropylidene-α-D-mannose furanose tert-butyl ester (Ⅰ) into a 250 mL three-necked flask, add 60 mL THF, stirred by magnetic force to dissolve, then protected by nitrogen, cooled in an ice-water bath to below 10°C (preferably between 0-10°C), then slowly added LiAlH40.03mol, raised the temperature and reacted at 15°C 4h, TLC monitors the entire reaction process (V petroleum ether: V ethyl acetate = 3:2), then slowly dropwise add 30mL ethyl acetate aqueous solution (V ethyl acetate: V water = 1:1) to end the reaction, filter the precipitate, Concentration under reduced pressure gave 4.338 g of a colorless transparent viscous liquid (II), with a yield of 88.95%.
[0039] (2) Preparation of 1-O-hydroxyethyl-D-mannopyranose (Ⅲ)
[0040] Add...
Embodiment 3
[0042] The preparation method of 1-O-hydroxyethyl-D-mannopyranose, it comprises the steps:
[0043] (1) Preparation of 1-O-hydroxyethyl-2,3:5,6-di-O-isopropylidene-α-D-mannofuranose (Ⅱ)
[0044]Weigh 6.0 g of 1-O-carboxymethyl-2,3:5,6-di-O-isopropylidene-α-D-mannofuranosyl tert-butyl ester (I) into a 250 mL three-necked flask, add 96 mL THF, stirred by magnetic force to dissolve, then protected by nitrogen, cooled in an ice-water bath to below 10°C (preferably between 0-10°C), then slowly added LiAlH40.033mol, raised the temperature and reacted at 25°C 5h, TLC monitored the entire reaction process (V petroleum ether: V ethyl acetate = 3:2), and then slowly dropwise added 40mL ethyl acetate aqueous solution (V ethyl acetate: V water = 1:1) to end the reaction, filtered the precipitate, Concentration under reduced pressure gave 4.2 g of a colorless transparent viscous liquid (II), with a yield of 86.12%.
[0045] (2) Preparation of 1-O-hydroxyethyl-D-mannopyranose (Ⅲ)
[0046...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com