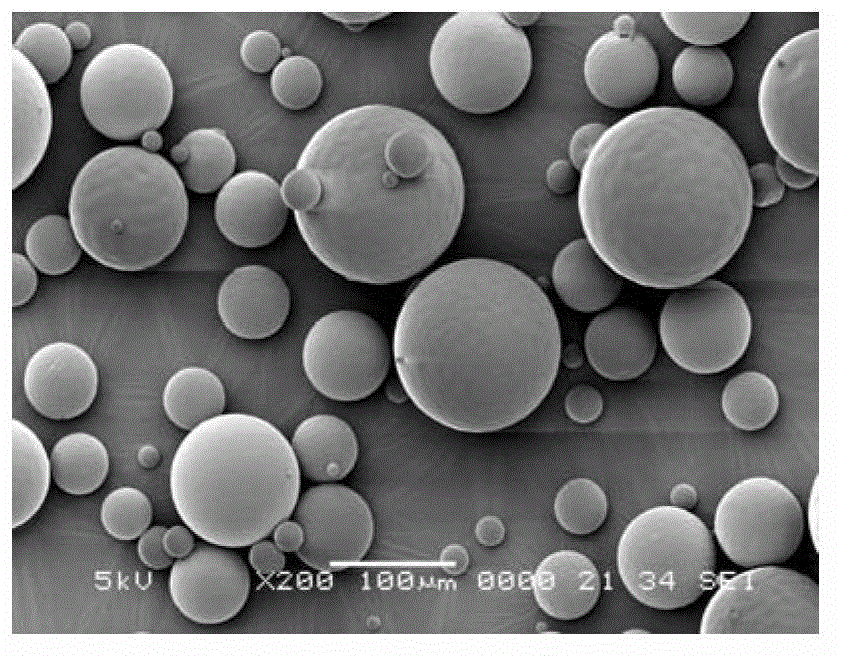
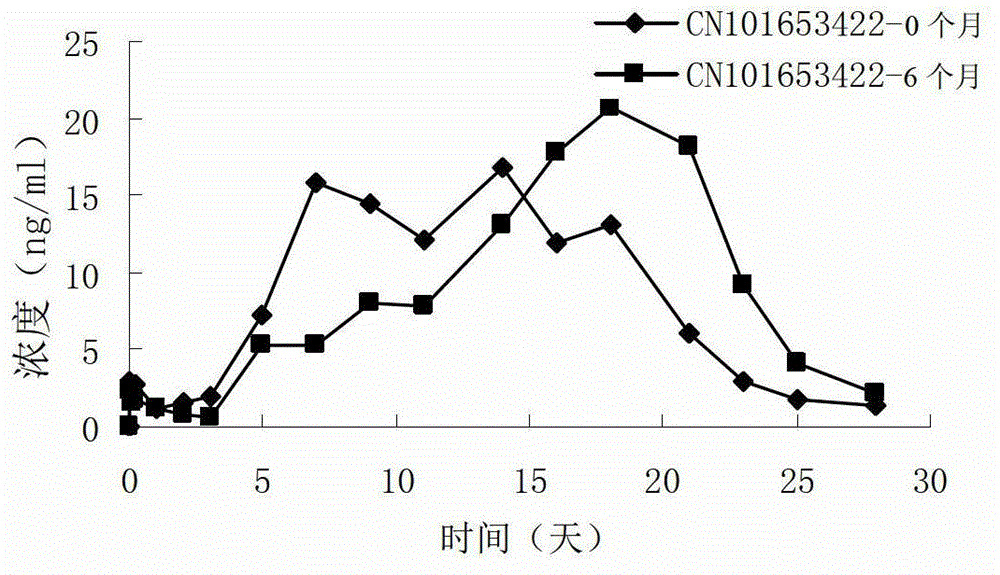
Risperidone sustained release microsphere composition
A composition and technology of risperidone, which are applied in the field of risperidone long-acting sustained-release microsphere composition and its preparation field, can solve the problems of changes in the release behavior of the microspheres, poor formulation stability and the like, so that the release behavior will not change. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Weigh 72g of PLGA with a molecular weight of 74,000 (75 / 25, 0.52A), 18g of PLGA with a molecular weight of 25,000 (50 / 50, 0.25A) and 110g of risperidone, stir and dissolve in 1000ml of dichloromethane to prepare Clear solution. This clear solution was added via a peristaltic pump to a microsphere preparation kettle containing 100 L of PVA solution (0.5%) cooled to 6 °C. The stirrer and homogenizer were turned on, and the clear solution was homogeneously emulsified at 380 rpm for 1 min. Afterwards, the rotation speed of the homogenizer is reduced, and the organic solvent is volatilized and removed for 3 to 5 hours. The residue was filtered through a mesh, washed with deionized water, and lyophilized to obtain the microspheres as a powder. No crystals were precipitated. The drug loading capacity of the microspheres was 45.9%, and the encapsulation efficiency was 83.5%.
Embodiment 2
[0052] Weigh 67.5g of PLGA with a molecular weight of 55,000 (75 / 25, 0.42A), 7.5g of PLGA with a molecular weight of 25,000 (50 / 50, 0.25A), and 75g of risperidone, stir and dissolve in 750ml of dichloromethane, A clear solution was prepared. This clear solution was added via a peristaltic pump to a microsphere preparation kettle containing 75 L of PVA solution (0.5%) cooled to 6 °C. The stirrer and homogenizer were turned on, and the clear solution was homogeneously emulsified at 380 rpm for 1 min. Afterwards, the rotation speed of the homogenizer is reduced, and the organic solvent is volatilized and removed for 3 to 5 hours. The residue was filtered through a mesh, washed with deionized water, and lyophilized to obtain powdered microspheres. No crystals were precipitated. The drug loading capacity of the microspheres was 40.2%, and the encapsulation efficiency was 80.4%.
Embodiment 3
[0054] Weigh 56g of PLGA with a molecular weight of 125,000 (75 / 25, 0.90A), 24g of PLGA with a molecular weight of 25,000 (50 / 50, 0.25A), and 120g of risperidone, stir and dissolve them in 1000ml of dichloromethane to prepare Clear solution. This clear solution was added via a peristaltic pump to a microsphere preparation kettle containing 100 L of PVA solution (0.5%) cooled to 6 °C. The stirrer and homogenizer were turned on, and the clear solution was homogeneously emulsified at 380 rpm for 1 min. Afterwards, the rotation speed of the homogenizer is reduced, and the organic solvent is volatilized and removed for 3-5 hours; the residue is filtered with a sieve, washed with deionized water, and freeze-dried to obtain powdered microspheres. No crystals were precipitated. The drug loading capacity of the microspheres was 51.5%, and the encapsulation efficiency was 85.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com