Cell strain K562 with specific expression of HLA-G1 antigen
A technology of HLA-G1 and cell lines, applied in the field of tumor cells, to achieve the effect of complete design and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
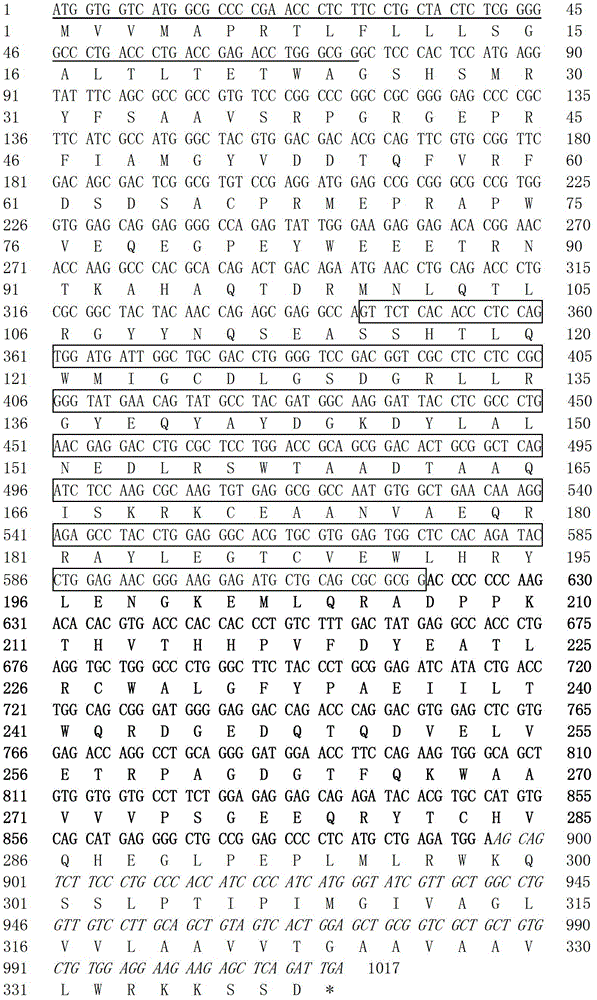
[0021] Example 1: Cloning of HLA-G1 gene and construction of pVITRO2-mcs-HLA-G1 recombinant plasmid
[0022] PCR amplification of the HLA-G1 gene sequences encoding restriction sites: design the following pair of primer sequences:
[0023] Upstream primers, the underlined part is EcoR I restriction site: 5'-TCGA GAATTC ATGGTG GTCATGGCGCCCCGAA-3'; (SEQ ID NO.1)
[0024] Downstream primers, the underlined part is xho I restriction site: 5'-TCGA CTCGAG TCAATC TGAGCTCTTCTT-3' (SEQ ID NO. 2).
[0025] Using the genome of human choriocarcinoma cell line JEG-3 as a template, HLA-G1 PCR amplification was carried out according to the following conditions, and the fragment size was 1037bp.
[0026] PCR system:
[0027] h 2 O: 50 μL
[0028] Buffer(10×): 5μL
[0029] Mg 2+(25mmol / L): 2μL
[0030] Primer-up (25μmol / L): 2μL
[0031] Primer-down (25μmol / L): 2μL
[0032] dNTP(20mmol / L): 1μL
[0033] Template (Genome of FRI 100, 10ng / μL):...
Embodiment 2
[0042] Example 2: Identification of K562 cell line stably expressing HLA-G1 antigen
[0043] RT-PCR was used to identify the mRNA expression of HLA-G1 in transfected cells: Trizol reagent was used to extract the total mRNA of each transfected cell line, and no degradation was identified by formaldehyde-denaturing agarose gel electrophoresis, A 260 / 280 The ratio is 2.0019. Take 2 μl of total mRNA and follow the instructions of Fermentas Reverse Transcriptase Kit to synthesize the first strand of cDNA. The parameters of the PCR reaction were: pre-denaturation at 94 °C for 4 min; 35 cycles of 1 min at 94 °C, 1 min at 60 °C, and 2 min at 72 °C; and a final extension at 72 °C for 10 min. Take 5μl of PCR product for agarose gel electrophoresis and observe the results. The specific bands amplified by RT-PCR conform to the expected target fragment length: HLA-G1 (1037bp) (such as Figure 4 shown). The results showed that the HLA-G1 expression-negative K562 cells successfully expr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com