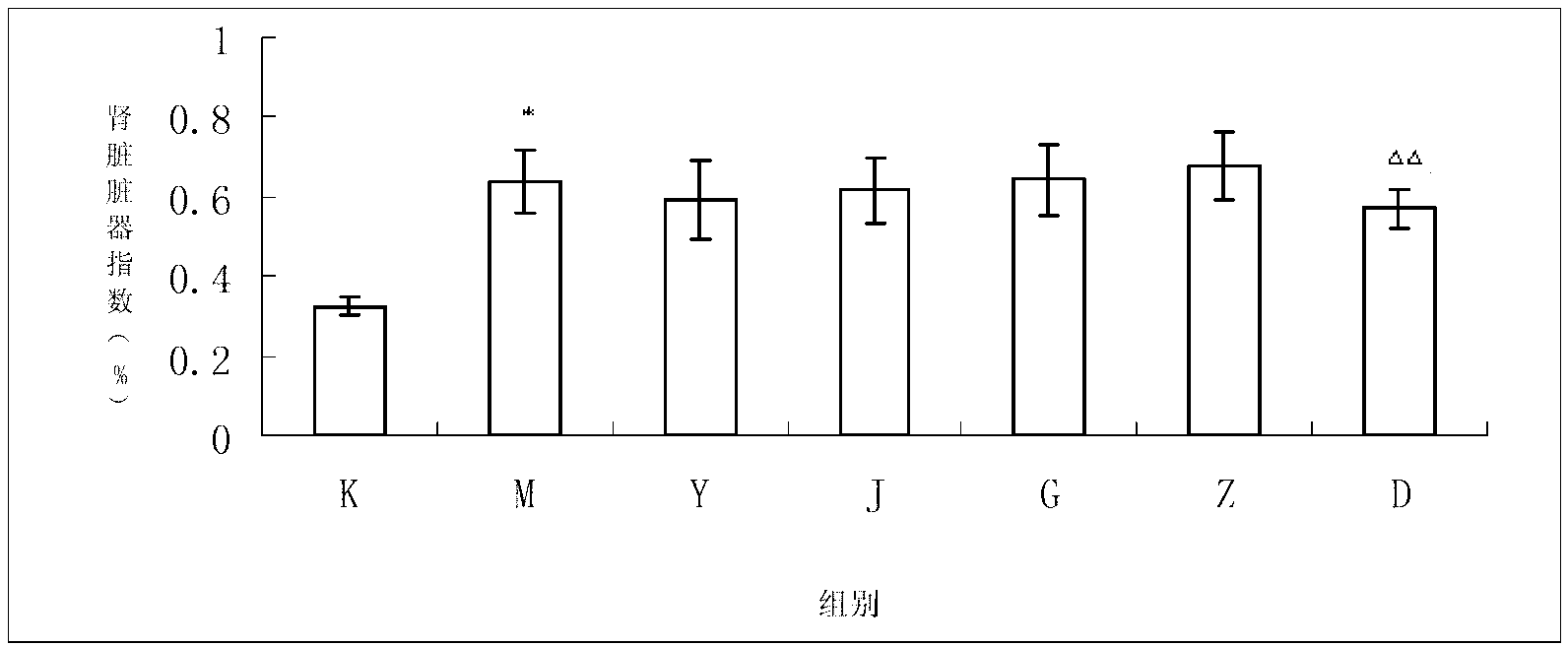Traditional Chinese medicine for treating diabetic nephropathy and preparation method thereof
A technology of diabetic nephropathy and traditional Chinese medicine, applied in the direction of medical formula, urinary system diseases, medical preparations containing active ingredients, etc., can solve the problem of no specific treatment for diabetic nephropathy, achieve the improvement of lipid metabolism disorder in the body, protect the structure of the kidney, reduce the Effect of urine protein content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
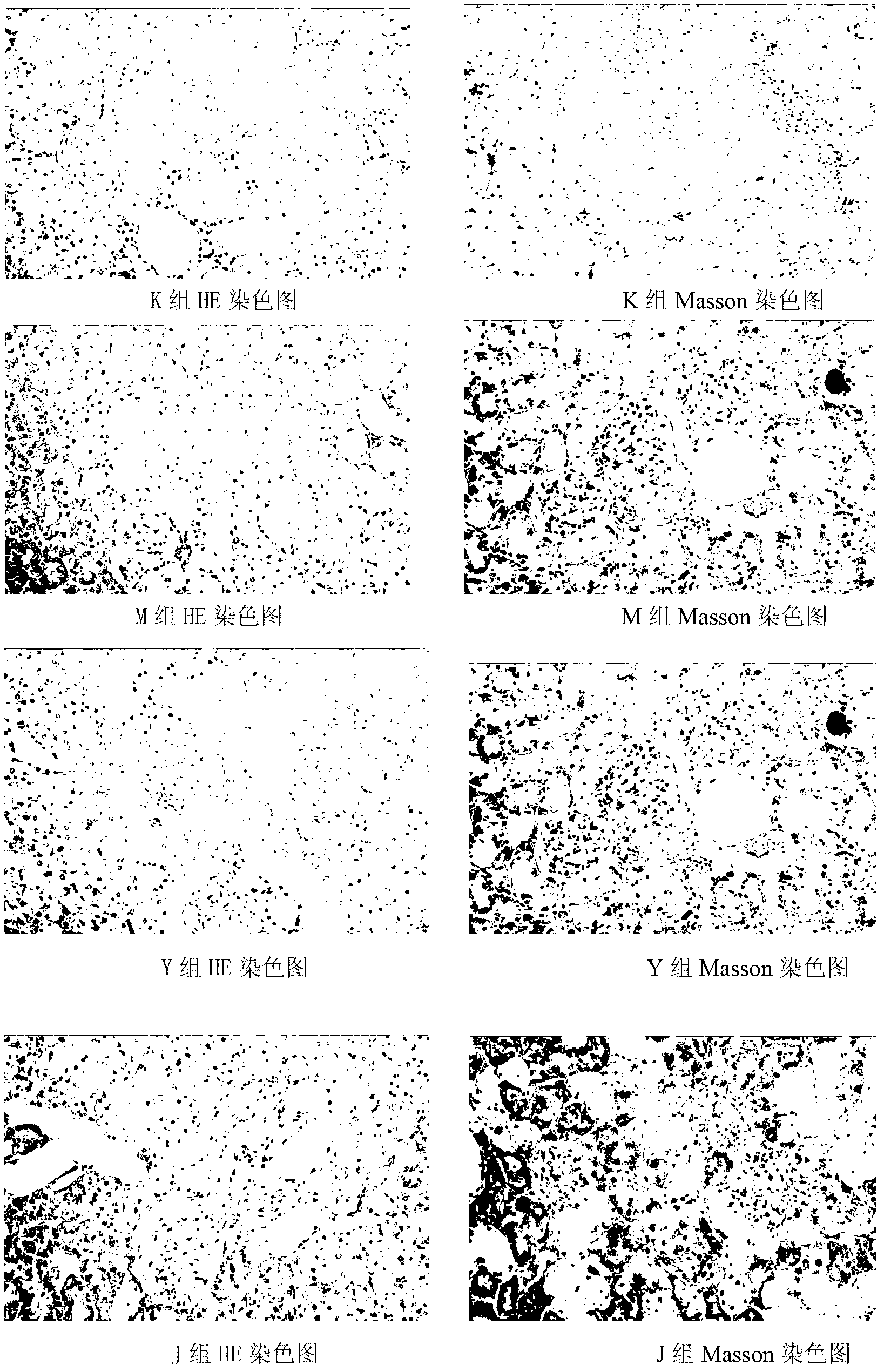
Image
Examples
Embodiment 1
[0029] A traditional Chinese medicine for treating spleen and kidney yang deficiency, which is made of the following Chinese medicinal materials in the weight ratio: 9 parts of fenugreek, 9 parts of astragalus, 5 parts of epimedium, 5 parts of psoralen, 4 parts of dogwood, cinnamon 4 parts, 3 parts of coptis, 2 parts of rhubarb.
[0030] The preparation method is as follows: take the Chinese medicinal materials according to the weight ratio, add astragalus by weight to 8 times of water to extract 3 times, each time for 1 hour, collect the extract and concentrate it to a clear paste with a relative density of 1.1; Add 6 times of 60% ethanol to reflux and extract twice, each time for 1 hour, collect the extract and concentrate it to a clear paste with a relative density of 1.1; Fenugreek, Epimedium, Psoralen, Cornus officinalis, and cinnamon are first weighed Add 8 times of water to extract three times, each time for 1 hour, collect the extract and concentrate to a clear paste w...
Embodiment 2
[0035] Example 2 Study on the therapeutic effect of the present invention on rats with type 1 diabetic nephropathy
[0036] 1. Test substance, reference substance, reagent
[0037] 1.1 Test product: the drug of Example 1, specification: 12g / bag; traits: this product is brown granular. Use an appropriate amount of double distilled water to prepare the concentration required for the experiment, and use it now.
[0038] 1.2 Reference substance 1: benazepril hydrochloride tablets. Source: Beijing Novartis Pharmaceutical Co., Ltd. Specifications: 10mg*14 tablets / box.
[0039] Control substance 2: Jin Kui Shen Qi Wan. Source: Beijing Tongrentang Co., Ltd. Tongrentang Pharmaceutical Factory. Specifications: 360 capsules / bottle.
[0040] Take Benazepril Hydrochloride Tablets and Jinkuishenqi Pills and prepare them with appropriate amount of double-distilled water to the concentration required for the experiment, and prepare them for immediate use.
[0041] 1.3 Reagents: STZ (st...
Embodiment 3
[0115] Example 3 The present invention's research on the therapeutic effect of type 2 diabetic nephropathy rats 1, test article, reference substance, solvent, reagent
[0116] 1.1 Test product: the drug of Example 1, specification: 12g / bag; traits: this product is brown granular. Use an appropriate amount of double distilled water to prepare the concentration required for the experiment, and use it now.
[0117] 1.2 Reference substance 1: benazepril hydrochloride tablets. Source: Beijing Novartis Pharmaceutical Co., Ltd. Specifications: 10mg*14 tablets / box.
[0118] Control substance 2: Jin Kui Shen Qi Wan. Source: Beijing Tongrentang Co., Ltd. Tongrentang Pharmaceutical Factory. Specifications: 360 capsules / bottle.
[0119] Take Benazepril Hydrochloride Tablets and Jinkuishenqi Pills and prepare them with appropriate amount of double-distilled water to the concentration required for the experiment, and prepare them for immediate use.
[0120] 1.3 Reagents: STZ (streptoz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com