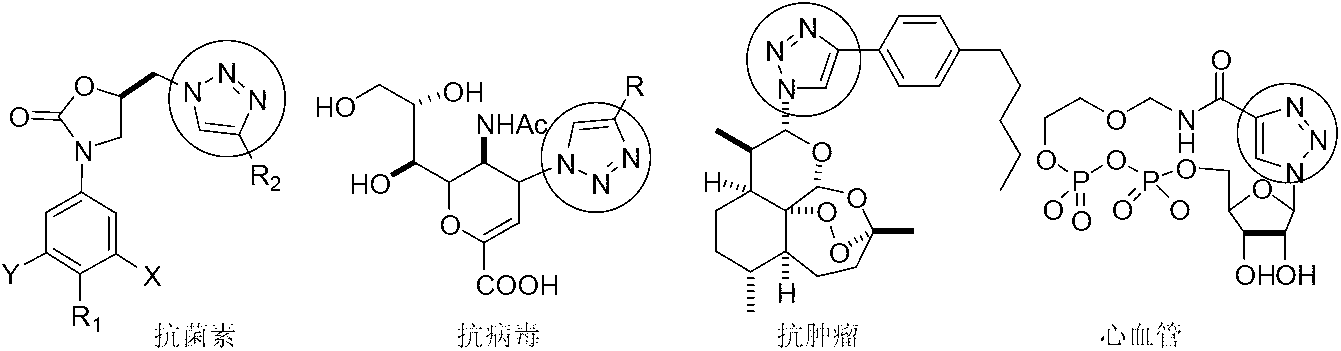
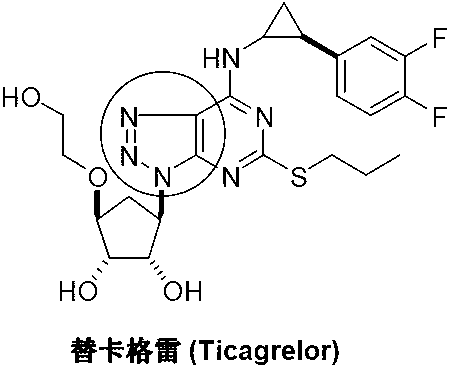
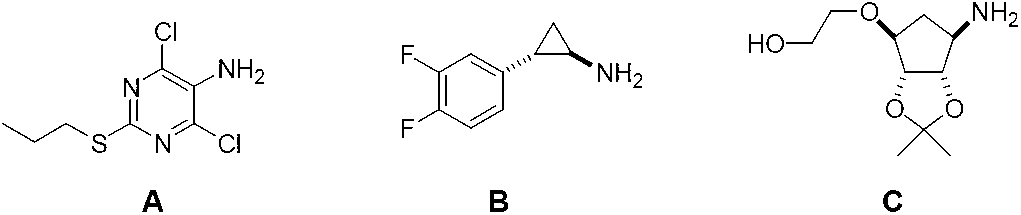
5-amino-1,4-disubstituent-1,2,3-triazole and preparation method thereof
A technology of disubstituted and triazoles, which is applied in the design of organic synthesis routes and the preparation of raw materials and intermediates, achieves the effects of cheap and easy-to-obtain raw materials, mild and easily controllable reaction conditions, and high product yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 0-5°C under a nitrogen atmosphere, add 2-cyanoacetamide (IIIa) (1.0g, 12mmol), sodium ethoxide (1.0g, 15mmol) and 20mL of absolute ethanol into the reaction flask, react for 30 minutes, add dropwise [3aR-(3aα, 4α, 6α, 6aα)]-6-azido-2,2-dimethyl-tetrahydro-4H-cyclopentadiene-1,3-dioxol-4 - Alcohol (II) (2.0 g, 10 mmol) in 20 mL ethanol. Slowly raise the temperature to co-flow, and keep the reflux reaction for 5 hours, and TLC detects that the reaction is complete. Cool to room temperature and filter to remove solids. The residue precipitated after adding water, filtered, and the solid was recrystallized with ethanol and ethyl acetate to obtain 1-[3aR-(3aα, 4α, 6α, 6aα)-[2,2-dimethyl-tetrahydro-4H- Cyclopenta-1,3-dioxol-4-ol]-6-yl]-5-amino-4-carboxamido-1,2,3-triazole (I) 2.4g , yield 84.8%.
Embodiment 2
[0030] 0-5°C under a nitrogen atmosphere, add 2-cyanoacetamide (IIIa) (1.0g, 12mmol), sodium methoxide (0.8g, 15mmol) and 20mL of anhydrous methanol into the reaction flask, react for 30 minutes, drop Add [3aR-(3aα, 4α, 6α, 6aα)]-[6-azido-2,2-dimethyl-tetrahydro-4H-cyclopentadiene-1,3-dioxol - 4-Oxy]ethanol (II) (2.4 g, 10 mmol) in 20 mL of methanol. Slowly rise to choke temperature, and keep at room temperature for 24 hours, and TLC detects that the reaction is complete. The solid was removed by filtration, and the residue was precipitated after adding water. After filtration, the product was recrystallized with isopropanol and ethyl acetate to obtain 1-[3aR-(3aα, 4α, 6α, 6aα)-[[2,2-dimethyl Base-tetrahydro-4H-cyclopentadiene-1,3-dioxol-4-oxyl]ethanol]-6-yl]-5-amino-4-carboxamido-1,2, 2.6 g of 3-triazole (I), yield 79.5%.
Embodiment 3
[0032] Add malononitrile (IIIc) (0.8g, 12mmol), potassium tert-butoxide (1.8g, 15mmol) and dry tetrahydrofuran solvent 25mL into the reaction flask under nitrogen atmosphere at 0-5°C, react for 30 minutes, drop Add [3aR-(3aα, 4α, 6α, 6aα)]-[6-azido-2,2-dimethyl-tetrahydro-4H-cyclopentadiene-1,3-dioxol -4-Oxy]methyl acetate (II) (2.7g, 10mmol) was dissolved in 30mL of tetrahydrofuran. Slowly rise to co-current, and maintain the reflux reaction for 6 hours, TLC detection of the completion of the reaction. The solid was removed by filtration, and the residue was precipitated after adding water. After filtration, the crude product was recrystallized with ethanol and ethyl acetate to obtain 1-[3aR-(3aα, 4α, 6α, 6aα)-[[2,2-dimethyl- Tetrahydro-4H-cyclopentadieno-1,3-dioxol-4-oxy]methyl acetate]-6-yl]-5-amino-4-cyano-1,2,3 - Triazole (I) 2.9g, yield 86.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com