Application of baicalin in preparation of medicine for treating polycystic ovarian syndrome
A polycystic ovary and baicalin technology, which is applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., can solve the problems of large side effects, unsatisfactory long-term effect, high recurrence rate, etc., and achieve the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
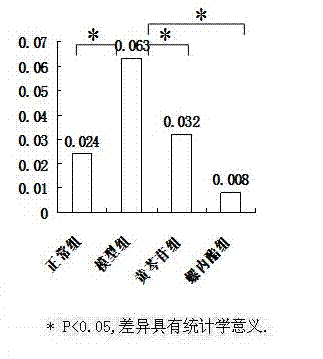
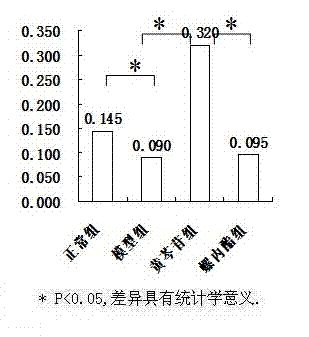
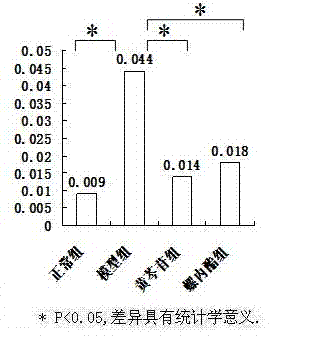
[0030] The animal model of polycystic ovary syndrome was prepared by using Wister rats, and the successful models were given drug intervention. ELISA, RT-PCR and other methods were used for detection, and statistical software SPSS16.0 was used for analysis to clarify the therapeutic effect and possible mechanism of baicalin on animal models of polycystic ovary syndrome.
[0031] 1. Materials
[0032] 1.1 Experimental animals
[0033] 190 21-day-old SPF-grade female Wister rats were provided by Shanghai Slack Experimental Animal Co., Ltd., and were bred in the Animal Center of the Sea Medicine Department of the Second Military Medical University. 25 ℃ constant temperature (50% humidity) clean-grade breeding, without feeding vitamin products, 12h light and 12h dark cycle alternately.
[0034] 1.2 Main reagents
[0035] Dehydroepiandrosterone (DHEA) was purchased from Sigma, sesame oil (for injection) was purchased from Sigma, baicalin (BAC) was purchased from Japan (TCI), spi...
Embodiment 2
[0072] NCI-H295R was selected as the model cell line for the in vitro study of polycystic ovary syndrome hyperandrogenism, and the androgen content of the cell supernatant was detected at a specific time through drug intervention at a specific concentration, and RT-PCR was used to detect the related genes. expression was identified.
[0073] 1. Materials and Methods
[0074] 1.1. Experimental cells
[0075] Due to the difficulty in obtaining clinical PCOS theca cells, and the adrenocortical carcinoma cell line (NCI-H295R) is internationally recognized as the best cell line for the study of androgen synthesis and metabolism, we chose adrenocortical carcinoma cells as experimental cells.
[0076] 1.2. Experimental reagents
[0077] Cell culture reagents DMEM / F12 medium and 0.25% trypsin were purchased from Pufei Biotech, newborn bovine serum (Nu-Serum I), ITS+PREMIX cytokines were purchased from BD Biosciences, adenylyl cyclase activator (Forskolin) was purchased from Sigma,...
Embodiment 3
[0096] Prescription: Take 2 g of baicalin, add 8 g of starch, 2 g of microcrystalline cellulose, and 0.2 g of magnesium stearate, mix well, and make tablets, capsules or granules by methods known in the art.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com