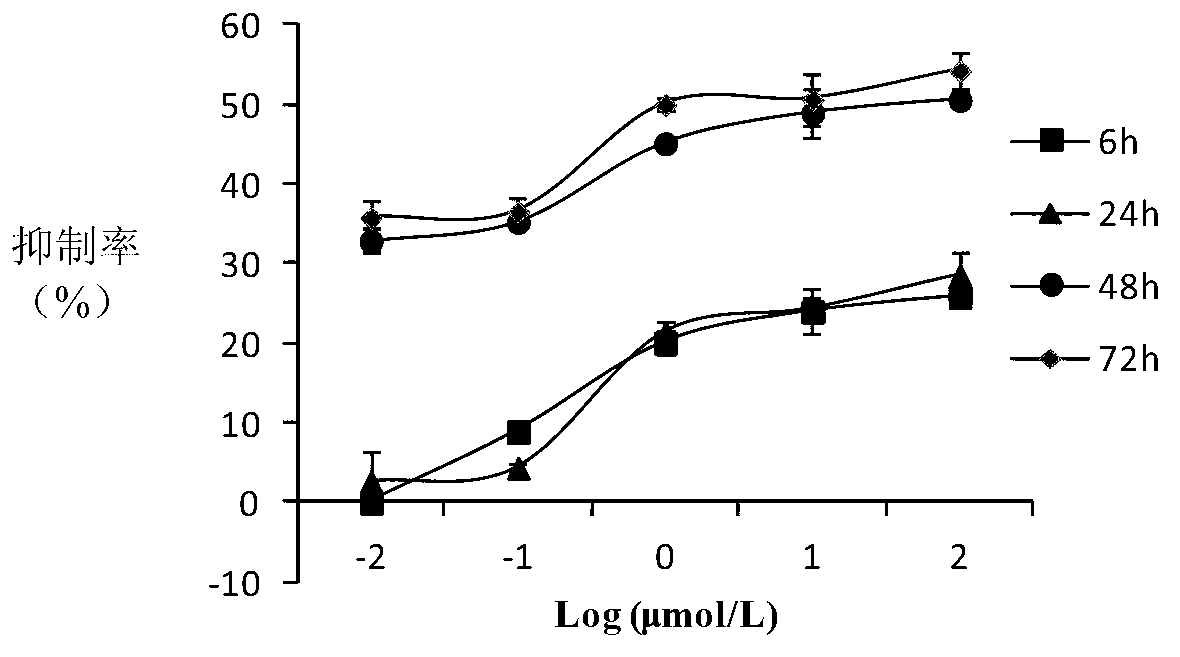
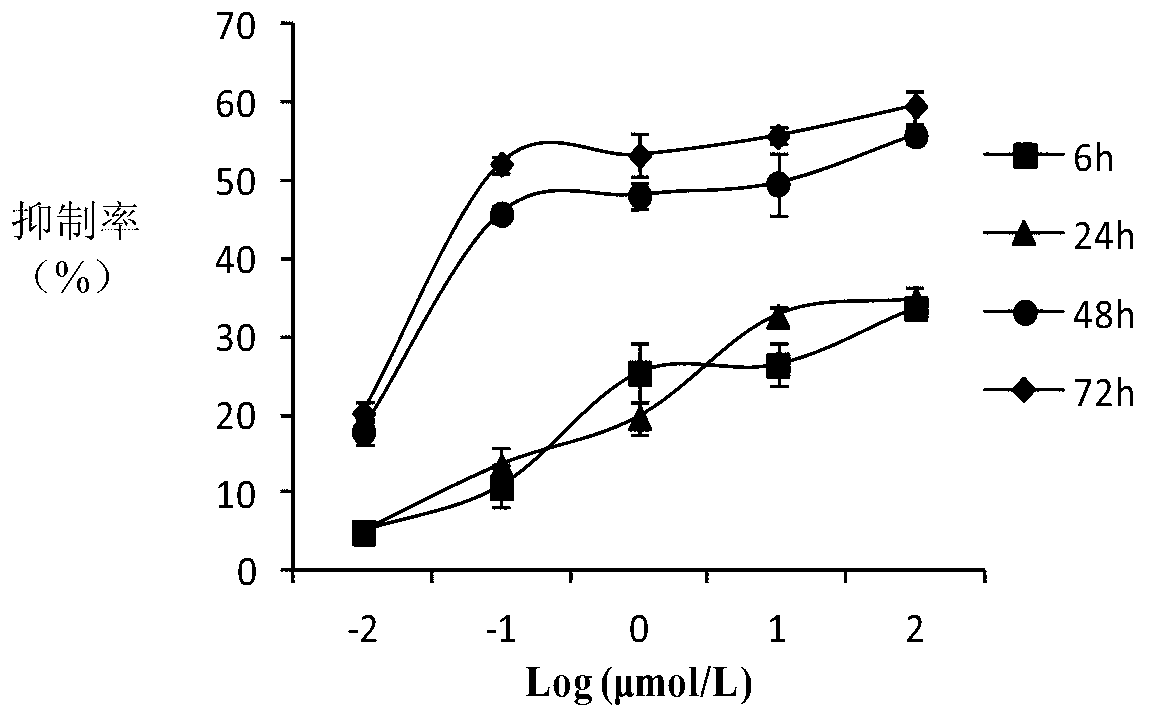
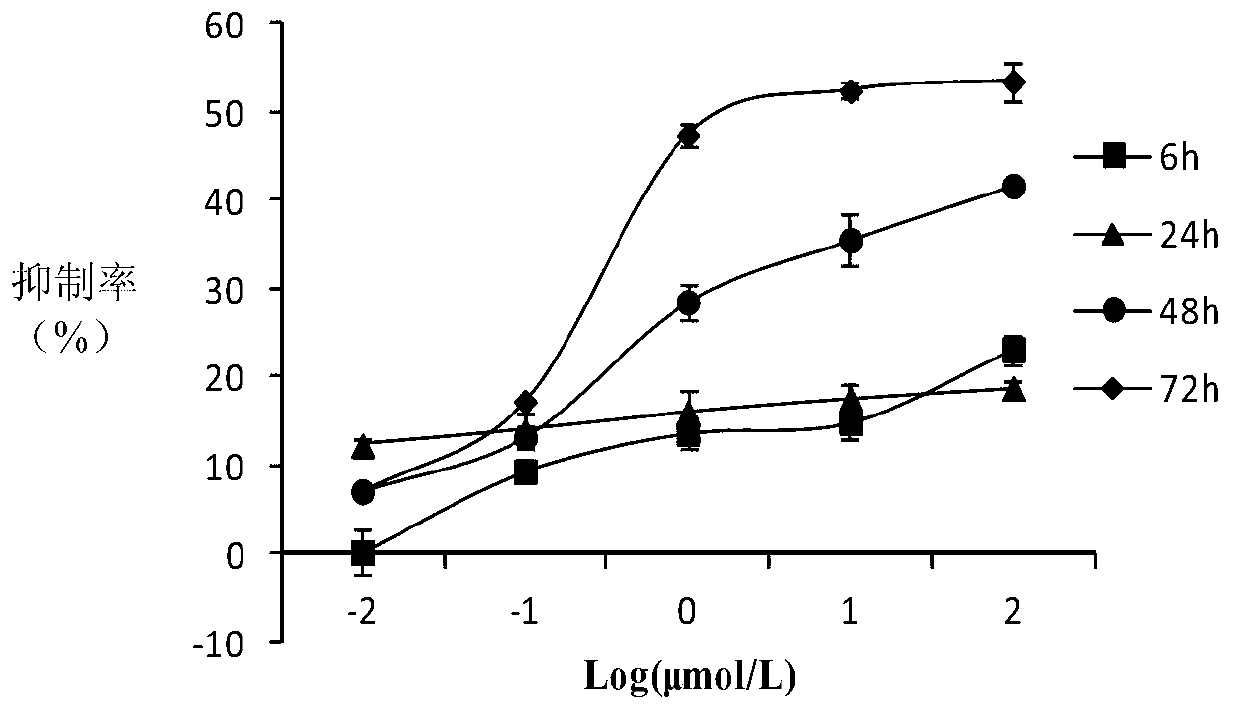
10-hydroxycamptothecine derivatives and applications thereof
A technology of hydroxycamptothecin and derivatives, applied in the field of pesticides, can solve the problems of the great influence of natural enemies' natural control ability, increase the dosage of drugs, environmental pollution, etc., and achieve the effect of excellent contact killing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] 10-[(2,2,3,3-Tetramethylcyclopropanyl)carboxylate]-(20S)-camptothecin (a) synthetic route: In a 250mL flask, add 10-hydroxycamptothecin ( 0.364g, 1mmol), the reaction bottle was pumped out of the air into the N 2 After that, 120 mL of tetrahydrofuran (THF), fenchromoyl chloride (0.96 g, 6 mmol) and triethylamine (Et 3 N) 0.9 mL. N 2 The reaction was stopped after reflux under protection for 4 hours. The reaction solution was transferred to a 500mL separatory funnel, 180mL ethyl acetate was added, and the mixture was evenly shaken. Then add 40 mL of distilled water, oscillate and mix completely, let stand to separate the liquid, take the upper organic phase, and repeat three times. The organic phase was transferred into a 500mL Erlenmeyer flask, and anhydrous sodium sulfate was added for shaking, left to stand, filtered, and the solution was rotary evaporated to obtain a light yellow solid. Add 20 mL of anhydrous diethyl ether to the solid, stir magnetically for 10 ...
Embodiment 2
[0064] 10-[[3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanyl]carboxylate]-(20S)-camptothecin (b) synthetic route: in a 250mL flask , added 10-hydroxycamptothecin (0.36g, 1mmol), the reaction flask was pumped out the air into N 2 Then, 120 mL of tetrahydrofuran, chrysanthemum acid chloride (1.35 g, 6 mmol) and 0.9 mL of triethylamine were added. N 2 The reaction was stopped after reflux under protection for 6 hours. The reaction solution was transferred to a 500mL separatory funnel, 180mL ethyl acetate was added, and the mixture was evenly shaken. Then add 40 mL of distilled water, oscillate and mix completely, let stand to separate the liquid, take the upper organic phase, and repeat three times. The organic phase was transferred into a 500mL Erlenmeyer flask, added anhydrous sodium sulfate to remove water, filtered and evaporated to dryness, and then passed through a silica gel column (dichloromethane / methanol / petroleum ether=70 / 0.5 / 30) to obtain 0.28g of a light yellow sol...
Embodiment 3
[0066] 10-[[3-(2-Chloro-2-trifluoromethylvinyl)-2,2-dimethylcyclopropanyl]formyl]-(20S)-camptothecin (c) synthetic route Similar to b, the column chromatography developing solvent is dichloromethane / methanol / petroleum ether=100 / 0.5 / 80), and finally 0.32 g of light yellow solid was obtained, with a yield of 55.1%. 1 H NMR (CDCl 3 ,300MHz)δ1.00(3H,t,J=7.4Hz,H-18),1.42,1.46(each3H,s,CH 3 ),1.89(2H,m,H-19),2.32(1H,d,J=8.3Hz,CHCOO),2.41(1H,t,J=8.7Hz,CHCHC(CF 3 )Cl), 4.39(1H,s,OH), 5.26(2H,s,H-5), 5.30(2H,s,H-17), 5.70(1H,d,CHC(CF 3 )Cl),6.95(1H,d,H-14),7.63(1H,s,H-11),7.71(1H,s,H-9),8.23(1H,d,J=9.2Hz,H- 12),8.30(1H,d,J=2.9Hz,H-7); 13 C NMR (CDCl 3 ,75MHz)δ7.7(CH 3 ,C-18),14.8(CH 3 ,2CH 3 ),28.3(C,C(CH 3 ) 2 ),28.8(CH 2 ,C-19),31.7(CH,CHCHC(CF 3 )Cl), 32.6(CH,CHCOO), 50.0(CH 2 ,C-5),66.2(CH 2 ,C-17),72.8(C,C-20),98.3(CH,C-14),114.9(CH,C-9),122.6(C,C-16,CHC(CF 3 ) Cl), 125.8, 128.4, 129.2, 130.5 (CH, C-7, 11, 12, CHC (CF 3 )Cl;C,C-6,8,CHC(CF 3) Cl), 146.0 (C, C-3), 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com