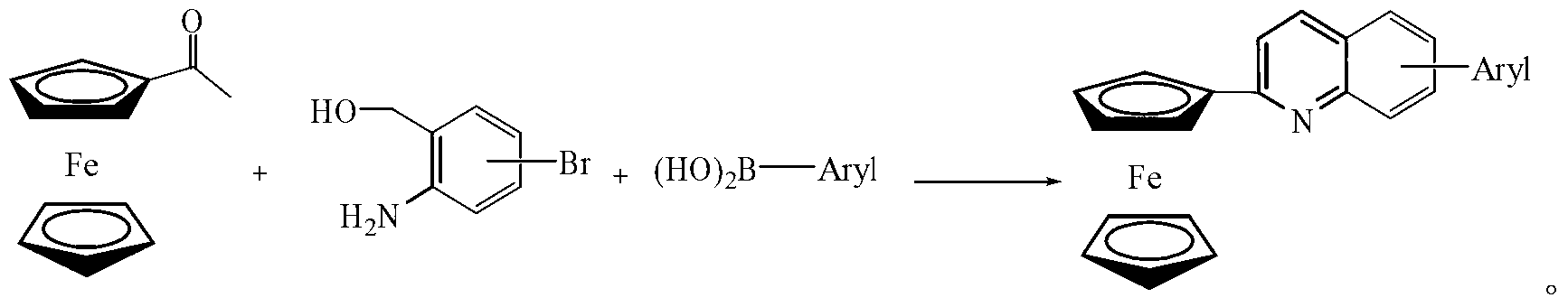2-ferrocenyl-arylquinoline and preparation method thereof
A ferrocene-based and aryl-quinoline technology, applied in the field of materials, achieves the effects of a wide range of substrates, mild reaction conditions, and low prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
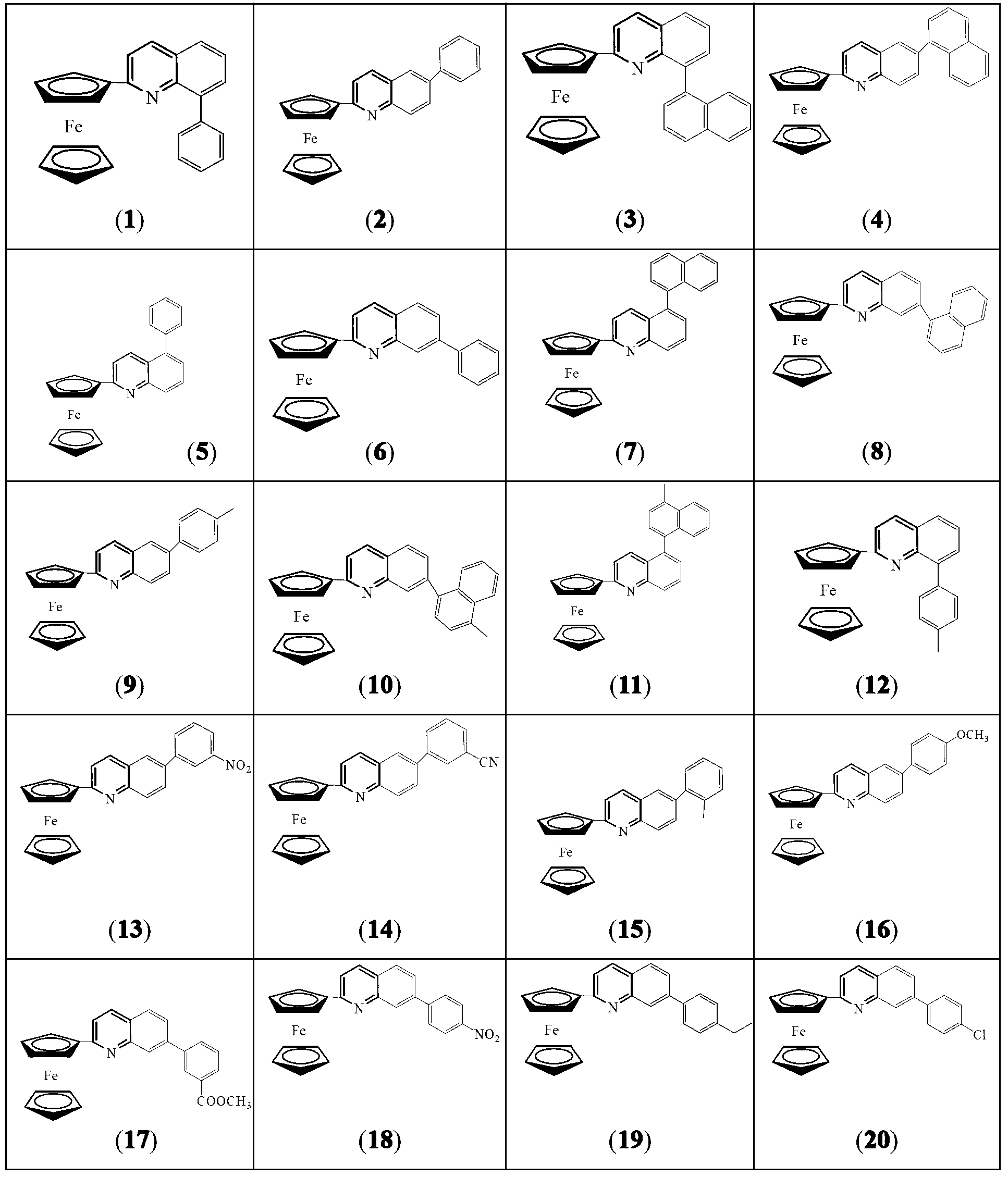
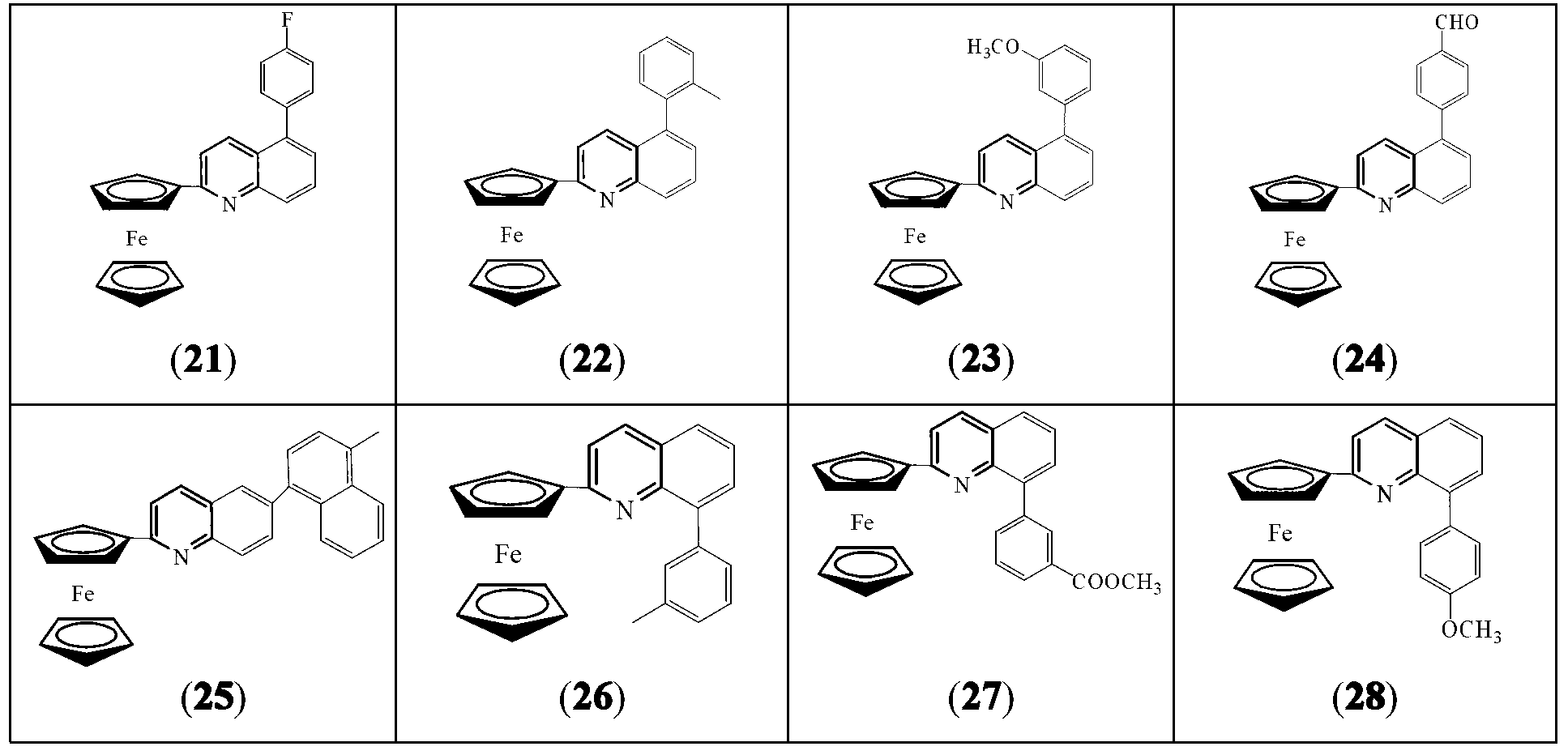
[0023] 2-ferrocenyl-arylquinoline, the general formula is:
[0024] The specific structure can be:
[0025]
[0026]
Embodiment 2
[0028] Compound (1) Preparation of 2-ferrocenyl-8-phenylquinoline: under the protection of high-purity nitrogen, add 1.0mmol acetylferrocene and 1.5mmol 6-bromo-o-hydroxymethyl to a 10ml Schlek reaction tube Aniline, 2.0mmol phenylboronic acid, 0.05mmol [Ir(cod)Cl] 2 , 0.05mmol palladium chloride, 0.15mmol triphenylphosphine, 3.0mmol sodium hydroxide and 5ml dioxane, replace the reaction tube with nitrogen for 3 times, then heat to 110°C with an oil bath under magnetic stirring, and reflux for 16 Hour.
[0029] The oil bath was removed, and the water bath was lowered to room temperature; 3ml of water was added to the reaction solution, extracted three times with 5ml of dichloromethane, the organic phases were combined and washed with anhydrous MgSO 4 Dry for 30 minutes, filter, and concentrate the filtrate with a rotary evaporator. The concentrated residue is separated by silica gel column chromatography using dichloromethane as a developing solvent to obtain pure product 1 w...
Embodiment 3
[0031] Compound (2) Preparation of 2-ferrocenyl-6-phenylquinoline: under the protection of high-purity nitrogen, add 1.0mmol acetylferrocene and 1.0mmol 4-bromo-o-hydroxymethyl to a 10ml Schlek reaction tube Aniline, 1.0mmol phenylboronic acid, 0.01mmol [Ir(cod)Cl] 2 , 0.01mmol palladium acetate, 0.05mmol triphenylphosphine, 1.0mmol potassium hydroxide and 5ml dioxane, replace the reaction tube with nitrogen for 3 times, then heat to 110°C with an oil bath under magnetic stirring, and reflux for 48 hours .
[0032] The oil bath was removed, and the water bath was lowered to room temperature; 3ml of water was added to the reaction solution, extracted three times with 5ml of dichloromethane, the organic phases were combined and washed with anhydrous MgSO 4 Dry for 30 minutes, filter, and the filtrate is concentrated by a rotary evaporator. The concentrated residue is separated by silica gel column chromatography using dichloromethane as a developing solvent to obtain pure produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com