Application of substance capable of reducing expression of zinc finger protein CTCF to preparation of drugs for treating leukemia
A technology of zinc finger protein and expression cassette, which is applied to the application field of substances that reduce the expression of zinc finger protein CTCF in the preparation of leukemia drugs, and can solve the problems of no obvious improvement in the cure rate of ALL and unclear pathogenesis of leukemia, so as to promote the occurrence of leukemia. Apoptosis, inhibition of apoptosis, effect of increasing sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1, RNA interference recombinant expression vector construction of zinc finger protein CTCF
[0053] 1. Selection of RNA interference target sequence
[0054] For the full-length cDNA sequence (SEQ ID No.6) of the zinc finger protein CTCF coding gene CTCF, the following three DNA sequences were selected as target sequences for RNA interference:
[0055] sh-1: Positions 809-827 of SEQ ID No.6 (ie 5'-TGACTGTACCTGTTGCTAC-3')
[0056] sh-2: 1103-1122 of SEQ ID No.6 (ie 5'-ATGTAGATGTGTCTGTCTAC-3')
[0057] sh-3: No. 1398-1416 of SEQ ID No.6 (ie 5'-TACTCGTCCTCACAAGTGC-3')
[0058] Positions 445-2628 in SEQ ID No.6 are open reading frames encoding the zinc finger protein CTCF shown in SEQ ID No.7.
[0059] 2. Small interfering RNA (siRNA)
[0060] Three siRNAs (siRNA-1, siRNA-2, and siRNA-3) were designed against the three target sequences of the zinc finger protein CTCF in step 1, respectively. The target sequence of siRNA-1 is sh-1, the target sequence of siRNA-2...
Embodiment 2
[0113] Example 2, RNAi gene knockout recombinant vector transfection of leukemia cells
[0114] 1. Cell culture for transfection
[0115] 24 hours before transfection, use RPMI-1640 medium containing 10% fetal bovine serum (FBS) in a 15ml culture dish at 37°C and 5% CO 2 The B-ALL cell line NALM-6 was cultured under the conditions and transfected when it reached 75%-90%.
[0116] 2. Transfection
[0117] The RNA interference recombinant expression vectors pDsU6-GFP-sh-1, pDsU6-GFP-sh-2, pDsU6-GFP-sh-3 and the vector pDsU6-GFP-sh-luc (RNAi Positive control, for silencing luciferase gene) respectively transfected the cells cultured in step 1 to obtain the recombinant leukemia cells NALM-6 / pDsU6-GFP-sh-1 containing RNA interference recombinant expression vector pDsU6-GFP-sh-1, Recombinant leukemia cell NALM-6 / pDsU6-GFP-sh-2 containing RNA interference recombinant expression vector pDsU6-GFP-sh-2, recombinant leukemia cell NALM-6 / pDsU6-GFP-sh-3 containing RNA interference recom...
Embodiment 3
[0120] Example 3, Western Blot Detection of Expression of Zinc Finger Protein CTCF in Recombinant Leukemic Cells
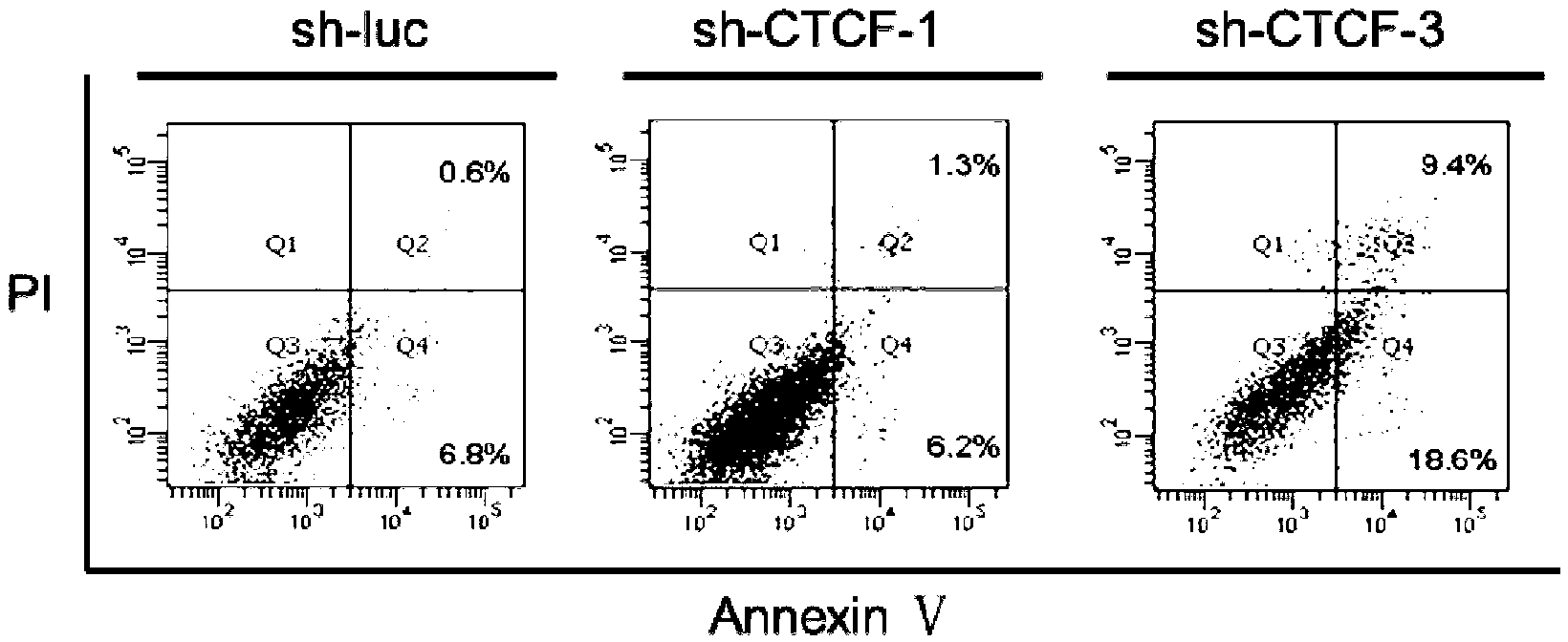
[0121] Take four kinds of recombinant leukemia cells NALM-6 / pDsU6-GFP-sh-1, NALM-6 / pDsU6-GFP-sh-2, NALM-6 / pDsU6-GFP-sh-3 after transfection 72h in Example 2 and NALM-6 / pDsU6-GFP-sh-luc, the total protein was extracted respectively, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as an internal reference for Western blot detection. The primary antibody for detecting zinc finger protein CTCF was CTCF( Molecular weight 83KDa) monoclonal antibody (purchased from Millipore), the primary antibody for detecting internal reference GAPDH is GAPDH (molecular weight 34KDa) monoclonal antibody (purchased from Shanghai Kangcheng Company in China), the results are as follows figure 1 As shown, the results show that: the recombinant leukemia cell NALM-6 / pDsU6-GFP-sh-1 containing RNA interference recombinant expression vector pDsU6-GFP-sh-1, and the recombinant expr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com