Sulfur-containing compound with nerve protection effect and application thereof
A neuroprotection and nerve cell protection technology, applied in the field of medicine, can solve problems such as weak inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 N-L-γ-glutamyl-S-coniferyl-L-cysteine and N-[N-L-γ-glutamyl-S-[3-(4-hydroxy-3-methoxyphenyl)-2-propenyl]-L-cysteinyl Preparation of ]-glycine
[0023] (1) Take 15kg of pineapple fruit, cut it into small pieces, mince it with a juice extractor, then extract it with 10L of 95% ethanol for 3 times, filter the part of the ethanol extraction with gauze, then centrifuge at 2000r / min, and recover under reduced pressure at 45°C Solvent until dry, weighed; then add an appropriate amount of deionized water, mix the sample with Amberlite XAD-16 at a ratio of 2:1 (dry ratio), and then apply Amberlite XAD-16 macroporous resin column chromatography, with different concentrations of ethanol Elution (H 2 O / EtOH, v / v, 1 / 0, 9 / 1, 4 / 1, 7 / 3, 3 / 2, 1 / 1, 2 / 3, 3 / 7 and 0 / 1), each eluted 6 column volume, respectively to obtain 9 (Fr.1-9) components. Among them, the component Fr.4 (H 2 O / EtOH, 7 / 3) by MCI column chromatography, with different concentrations of methanol / water (v / v, ...
Embodiment 2
[0033] Embodiment 2 Preparation of N-L-γ-glutamyl-S-sinapyl-L-cysteine and S-sinapylglutathione
[0034] (1) Pineapple extraction, macroporous resin segmentation, MCI and Sephadex LH-20 separation are the same as in Example 1. The difference is that the component Fr.2'' is further separated by preparative HPLC.
[0035] (2) Preparative liquid chromatography separation conditions
[0036] Instrument: Waters600 preparative liquid chromatograph equipped with 2487 dual-wavelength detector.
[0037] Chromatographic column: Phenomenex Luna C18 (2) column (250×21.2 mm, 5 μm).
[0038] Mobile phase: CH 3 CN-H 2 O (v / v, 17 / 83); detection wavelength: 254nm. The chromatographic peaks at 23.7 and 28.3 minutes were collected respectively, and the solvent was recovered to obtain N-L-γ-glutamyl-S-sinapyl-L-cysteine and S-sinapylglutathione.
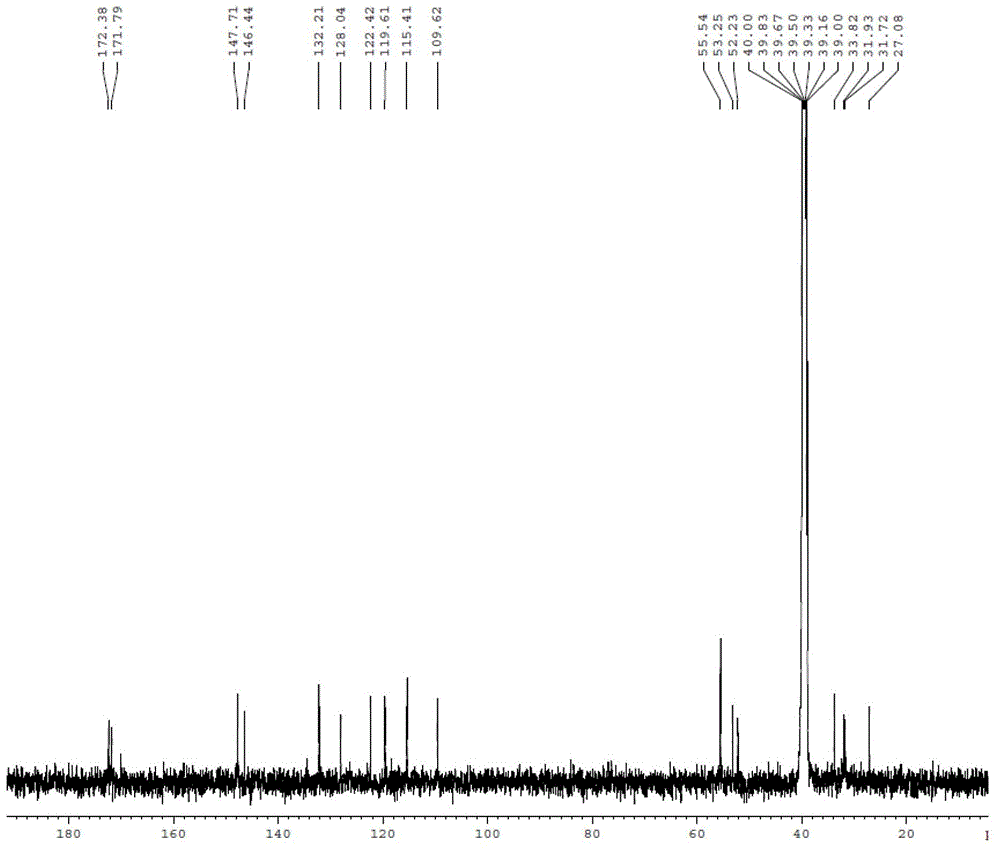
[0039] (3) NMR data and mass spectrometry data of N-L-γ-glutamyl-S-sinapyl-L-cysteine are as follows:
[0040] 1 H NMR (500MHz, DMSO-d 6...
Embodiment 3
[0043] The preparation of embodiment 3S-sinapyl-L-cysteine
[0044] (1) Pineapple extraction, macroporous resin segmentation, MCI and Sephadex LH-20 separation are the same as in Example 1. The difference is that the component Fr.3'' is further separated by preparative HPLC.
[0045] (2) Preparative liquid chromatography separation conditions
[0046] Instrument: Waters600 preparative liquid chromatograph equipped with 2487 dual-wavelength detector.
[0047] Chromatographic column: Phenomenex Luna C18 (2) column (250×21.2 mm, 5 μm).
[0048] Mobile phase: CH 3 CN-H 2 O (v / v, 15 / 85); detection wavelength: 254nm. The chromatographic peak at 21.3 min was collected, and the solvent was recovered to obtain S-sinapyl-L-cysteine.
[0049] (3) The nuclear magnetic resonance data and mass spectrometry data of S-sinapyl-L-cysteine are as follows:
[0050] 1 H NMR (500MHz, DMSO-d 6 ): δ6.80 (2H, s, H-2, 6), 6.40 (1H, d, J=15.6Hz, H-7), 6.10 (1H, d t, J=15.6, 7.3Hz, H-8) , 3.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com