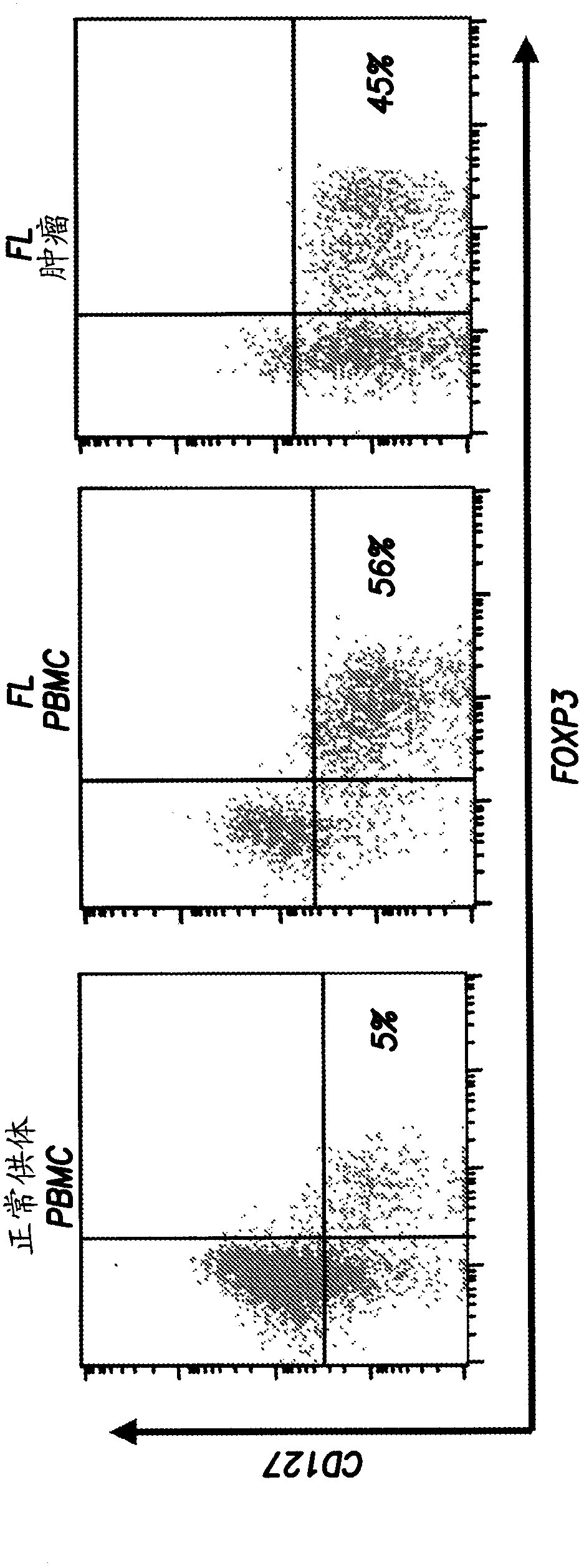
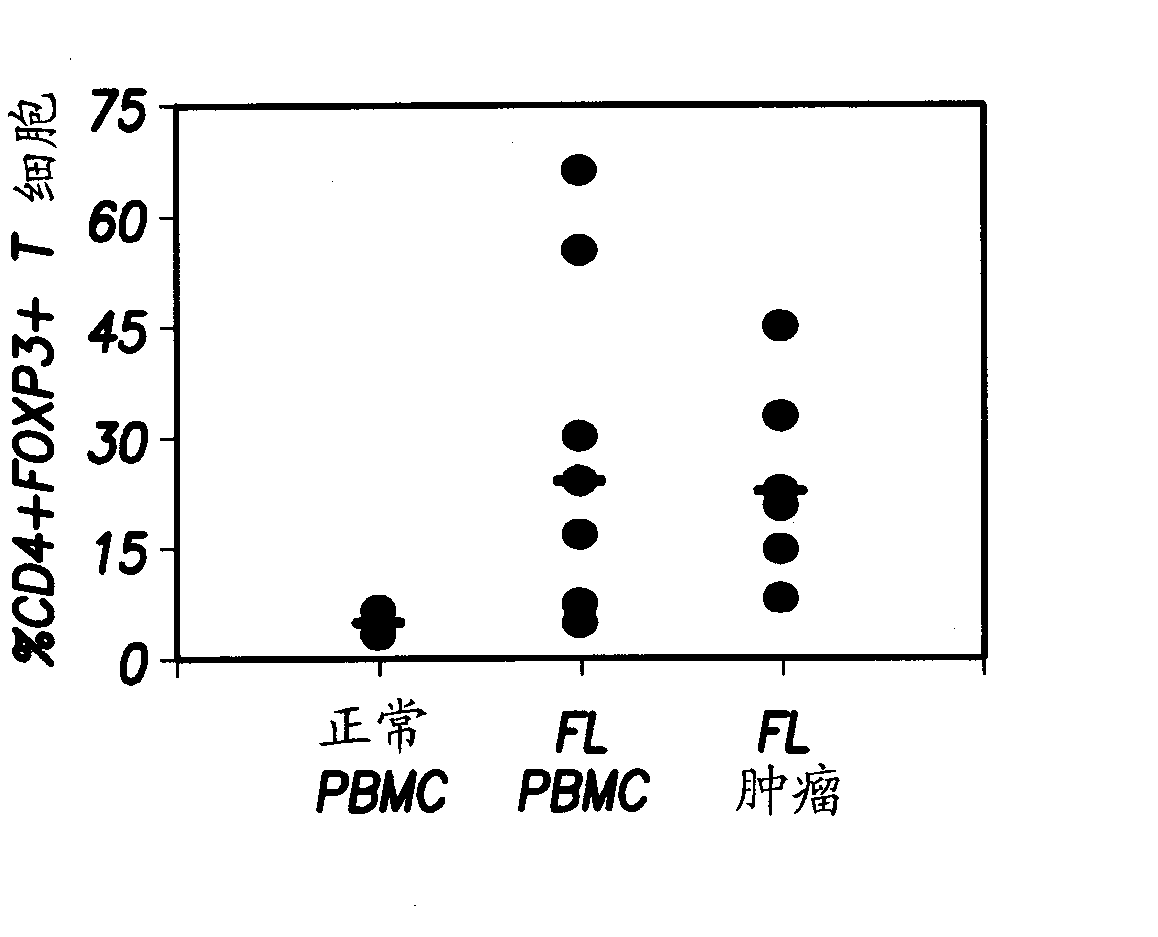
Anti-OX40 antibodies and methods of using the same
An antibody and sequence technology, applied in the field of regulation of OX40 receptor activation, can solve the problems of little known molecular signals and elusive signals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0206] Chimeric and humanized 106-222 IgG1 / κ mAbs (Ch222 and Hu222, respectively) were purified from the culture supernatants of the corresponding NSO stable transfectants using protein A columns as described in Appendices A and B . Hu222 was eluted from the column in two different ways. Briefly, Hu222 lot I was eluted with low pH buffer and lot II with Pierce's Gentle Ag / Ab elution buffer. Yield of Hu222 is better when eluted with lower pH buffer. Ch222 was eluted from the column with Gentle Ag / Ab Elution Buffer.
[0207] Purified Hu222 batches I and II antibodies were characterized by SDS-PAGE along with mouse 106-222 according to standard procedures. 5 μg of each antibody was analyzed under reducing conditions. Such as Figure 21 As shown, the Hu222 Lot I and II antibodies each consist of a heavy chain with a molecular weight of about 50 kD and a light chain with a molecular weight of about 25 kD. The purity of the Hu222 Lot I and II antibodies was shown to be over 95%....
Embodiment II
[0235] Purification of Ch119-122 and Hu119-122 Antibodies
[0236] The chimeric 119-122 IgG1 / κ monoclonal antibody (Ch119) was purified from the culture supernatant of the corresponding NSO stable transfectants (clone G11) grown on Hybridoma-SFM medium (Invitrogen) using a protein A column. After elution with Pierce's Gentle Ag / Ab Elution Buffer, Ch119 was buffer exchanged into PBS by gel filtration followed by dialysis. The concentration of Ch119 was 0.21 mg / ml.
[0237] Humanized 119-122 IgG1 / κ monoclonal antibody (Hu119) was purified from the culture supernatant of the corresponding NSO stable transfectants (clone 2F5) grown on Hybridoma-SFM medium (Invitrogen) using a protein A column. Hu106-222 was eluted from the column with a low pH buffer, neutralized with 1M Tris-HCl (pH 8.0) and dialyzed against PBS. The concentration of Hu122 was 1.6 mg / ml.
[0238] Purified Hu106-222 was characterized by SDS-PAGE along with mouse 119-122 according to standard procedures. 5 μg o...
Embodiment III
[0243] To assess the ability of our humanized anti-human OX40 antibody to enhance T cell proliferation, we used anti-CD3-coated CD32-L cells and freshly sorted naive CD4 + T cells were subjected to proliferation assays. Figure 27 Humanized anti-human OX40 mAb clone 119-122 (Hu122) and its FcR-binding mutant antibody (Hu122-AA) were shown to enhance naive CD4 +T cell proliferation. Compared with the parental mouse anti-human OX40 mAb (Mouse122), Hu122 produced better T cell stimulatory activity ( Figure 27 ).
[0244] FcR-binding mutant humanized anti-human OX40 mAb clone 106-222 (Hu222-AA) and chimeric anti-human OX40 mAb clone 106-222 (Ch222) enhance anti-CD3-stimulated naive CD4 + T cell proliferation. These antibodies have similar stimulatory activity compared to the parental mouse anti-human OX40 mAb (Mouse106-222). However, the fully humanized anti-human OX40Ab, Hu106, did not enhance T cell proliferation ( Figure 28 ).
[0245] To evaluate the blocking of CD4 b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com