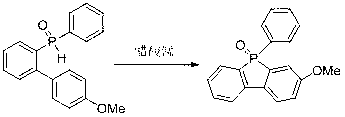
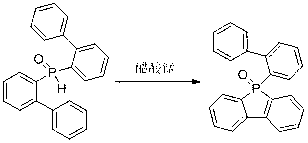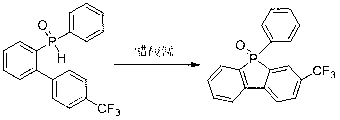Method for preparing dibenzophosphole derivants
A technology for heterocyclopentadiene and derivatives, which is applied in the field of preparation of dibenzophosphine derivatives, and can solve the problem of restricting the industrial production of dibenzophosphine derivatives and reaction substrates. Difficult to obtain, small scope of application, etc., to achieve the effect of diverse product types, short reaction time, and universality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Synthesis of 9-methyl-phosphorene-9-oxide
[0026]
[0027] Add (biphenyl-2-yl)-methylphosphine oxide (0.22 g, 1 mmol), manganese acetate (0.27 g, 1 mmol), ethanol (10 mL) into the reaction flask, react at 40 ° C, and track with TLC The reaction was carried out until the end; the crude product obtained after the reaction was separated by column chromatography to obtain the target product (yield 78%). Main NMR test data: 1 H NMR (400 MHz, CDCl 3 ): δ7.28-7.79 (m, 8H), 3.40 (s, CH 3 ), it can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
Embodiment 2
[0028] Example 2: Synthesis of (9-biphenyl-2-yl)-phosphorene-9-oxide
[0029]
[0030] Add bis(biphenyl-2-yl)-phosphine oxide (0.35 g, 1 mmol), manganese acetate (0.41 g, 1.5 mmol), and acetic acid (10 mL) into the reaction flask, react at 20°C, and track the reaction by TLC Until the end; the crude product obtained after the reaction was separated by column chromatography to obtain the target product (yield 76%).
[0031] Main NMR test data: 1 H NMR (400 MHz, CDCl 3 ): δ8.60-8.72 (m, 1H), 7.45-7.57 (m, 4H), 7.20-7.33 (m, 6H), 7.01-7.02 (m, 1H), 6.85 (t, J = 7.0Hz, 1H), 6.60 (t, J = 7.0Hz, 2H), 6.24 (d, J = 7.0Hz, 2H); 13 C NMR (100MHz, CDCl 3 ): δ142.3 (d, J = 22.2Hz), 137.0 (d, J = 402.6Hz), 133.0, 132.1, 131.3 (d, J = 9.8Hz), 129.4 (d, J = 9.2Hz), 129.0 (d, J = 10.8Hz), 128.9, 127.6 (d, J= 10.8Hz), 127.1, 126.6, 121.5 (d, J =9.8Hz). HRMS (M + ): m / z (%), calcd for C 24 h 17 OP 352.1017, found 352.1005 (M + , 61.65), the analysis shows that...
Embodiment 3
[0032] Example 3: Synthesis of 9-phenyl-phosphorene-9-oxide
[0033]
[0034] (Biphenyl-2-yl)-phenylphosphine oxide (0.28 g, 1 mmol), manganese acetate (0.41 g, 1.5 mmol), acetonitrile (10 mL) were added to the reaction flask, and the reaction was carried out at 20 °C, followed by TLC The reaction was carried out until the end; the crude product obtained after the reaction was separated by column chromatography to obtain the target product (yield 85%).
[0035] Main NMR test data: 1 H NMR (400 MHz, CDCl 3 ): δ7.24-7.81 (m, 13H); 13 C NMR (100MHz, CDCl 3 ): δ142.0 (d, J = 21.8Hz), 133.6 (d, J = 2.0Hz), 133.0 (d, J = 106.7Hz), 132.4 (d, J = 2.9Hz), 131.2 (d, J = 10.4Hz), 131.0 (d, J = 103.2Hz), 130.1 (d, J = 9.6Hz), 129.7 (d, J = 9.6Hz), 128.9 (d, J = 12.6Hz), 121.4 (d, J = 10.1Hz). HRMS (M + ): m / z (%), calcd for C 18 h 13 OP 276.0704, found 276.0706 (M + , 62.04) , it can be seen from the analysis that the actual synthesized product is consis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com