Bone generation by gene therapy
A technology for osteoblasts and chondrocytes, applied in gene therapy, genetic engineering, osteoblasts, etc., can solve problems such as short duration and large amounts of recombinant proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] Embodiment 1—Experimental steps of bone regeneration
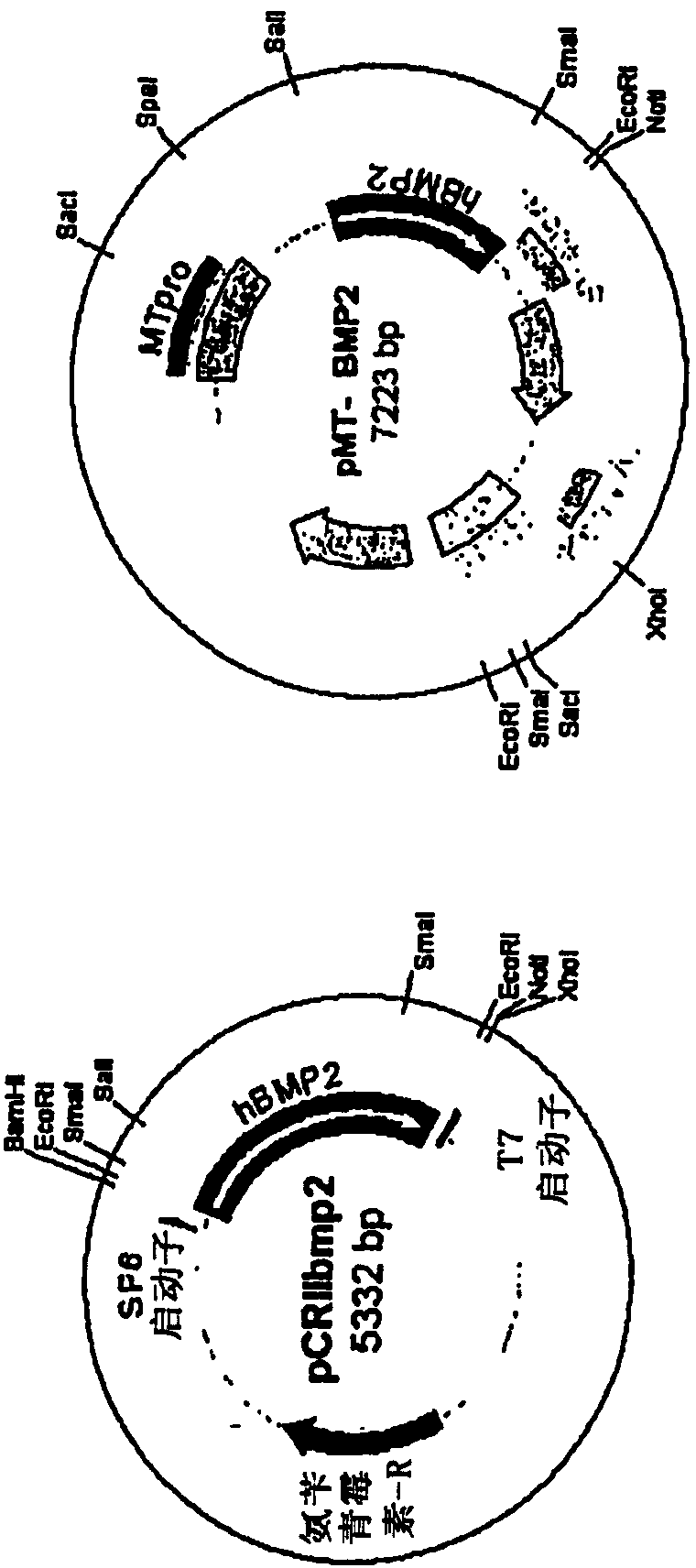
[0139] The human BMP2 gene was cloned by PCR (polymerase chain reaction) using human fetal brain cDNA and two primers. The 5' primer was 5'-TCCCAGCGTGAAAAGAGAGACTGC-3' (SEQ ID NO: 1), and the 3' primer was 5'-TTTTGCTGTACTAGCGACACCCACAACC-3' (SEQ ID NO: 2). After utilizing the GC-rich PCR system (Roche), the TOPO TA cloning kit (Invitrogen) was used to clone into the pCRII-TOPO vector ( figure 1 A). For cloning into retroviral vectors, pCRIIbmp2DNA was cut with Sal I and the human BMP2 cDNA insert was ligated into pMTMLV with Sal I and Not I overhangs ( figure 1 B). The packaging cell line GP-293 cells (5x10 5 cells / p60 dish). GP-293 cells were transfected with pMTMLV or pMT-BMP2 using Fugene (Roche). 48 hours after transfection, neomycin was added to the medium for selection of neomycin-resistant cells. Screening lasted 10 days. 293MT and 293MTBMP2 cells (5x10 5 cells / p60 dish) was used for transfection of ...
Embodiment 2
[0140] Example 2—Injection of NIH3T3-BMP-2 cells into rabbits
[0141] New Zealand white rabbits weighing 2.0-2.5 kg were selected for animal studies. The tibia was exposed and a defect (2 cm long and 0.5 cm deep) was created with orthopedic instruments. After suturing, control NIH3T3-neo or NIH3T3-BMP-2 cells (2ml2x10 6 cells / ml) were injected into the defect site. Eight weeks after injection of the cells, radiological analysis and histological examination were performed.
Embodiment 3
[0142] Example 3 - weekly X-ray examination
[0143] New Zealand white rabbits weighing 2.0-2.5 kg were selected for animal studies. The tibia was exposed and a defect (2 cm long and 0.5 cm deep) was created with orthopedic instruments. After suturing, NIH3T3-BMP-2 cells (2ml2x10 6 cells / ml) were injected into the tibial defect site. Radiographic analysis was then performed at 1, 2, 3, 4, 5, 6 and 7 weeks after cell injection. Samples were harvested 7 weeks after injection and plotted. Histological examination was performed after harvest.
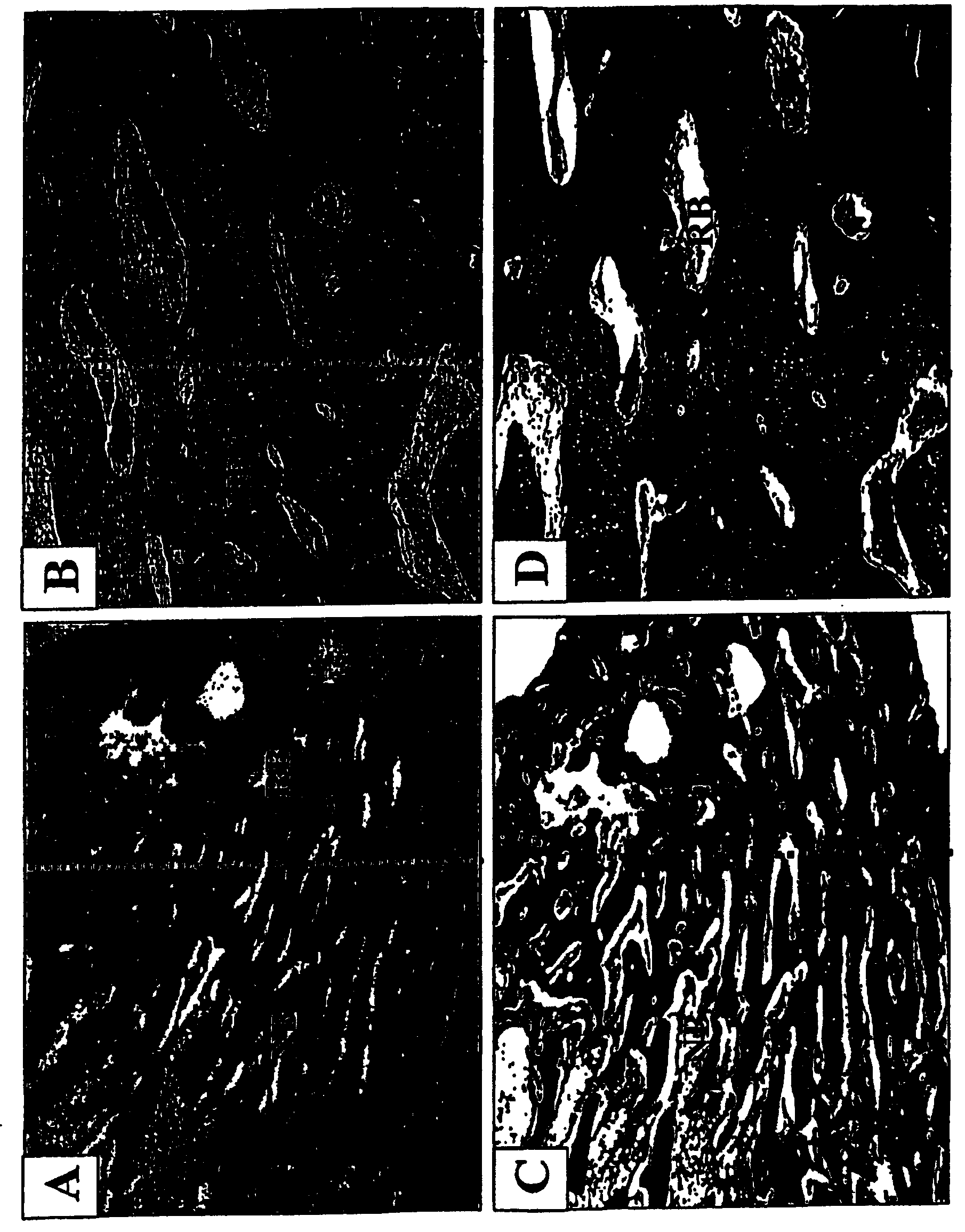
[0144] figure 2 A-2F show bone regeneration with NIH3T3-BMP-2 fibroblasts. figure 1 A and 1B show images of leg bones 8 weeks after injection of control NIH3T3 fibroblasts (A) and NIH3T3-BMP-2 cells (B). figure 2 C-2F shows radiographs of control (C&D) and experimental (E&F) leg bones before sacrifice of animals. Bone defects treated with cells expressing the BMP-2 protein healed 8 weeks after injection, whereas no bone regenerati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com