Compound, and preparation method and application thereof
A technology of compound and alkyl, applied in the field of compound and its preparation, can solve the problem of loss of physiological activity and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
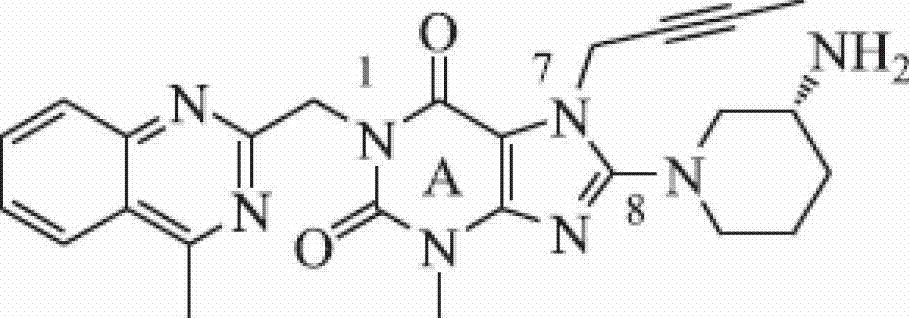
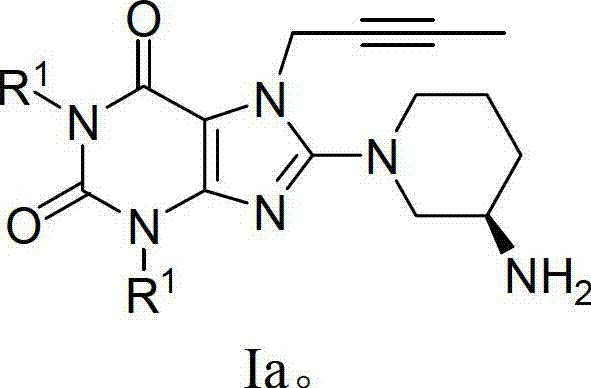
[0145] Example 1: Compound 1 (1,3-bis[(quinolin-2-yl)-methyl]-7-(2-butyn-1-yl)-8-[(R)-3-amino- Preparation of piperidin-1-yl]-xanthine)
[0146] a. Preparation of intermediate 8-bromoxanthine
[0147] Take 15g of xanthine, add 250mL of water and 7.6mL of bromine, heat to 100°C, stop the reaction when the color of bromine disappears, cool the reaction solution to room temperature, filter with suction, wash the filter cake with a small amount of water, and dry in vacuo to obtain product 18.4 g;
[0148] b. Intermediate 7-(2-butyn-1-yl)-8-bromoxanthine
[0149] Weigh 2.3g of 8-bromoxanthine and dissolve it in 30mL DMF, then add 1.75mL DIPEA and 0.9mL 1-bromo-2-butyne successively, heat the reaction solution to 80°C, stop the reaction after 17h, cool to room temperature, and then add 50 mL of water was added to the reaction solution, and a white solid was precipitated, which was filtered with suction, and the filter cake was washed with a small amount of water, and dried in vac...
Embodiment 2
[0157] Example 2: Compound 2(1,3-bis(2-cyanobenzyl)-7-(2-butyn-1-yl)-8-[(R)-3-amino-piperidine-1- base]-xanthine) preparation
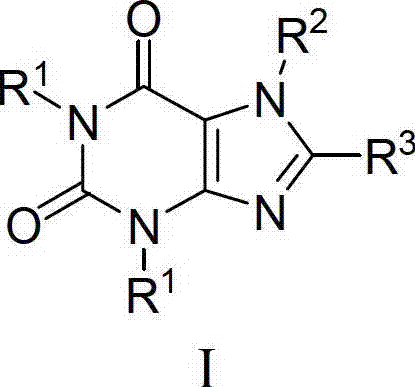
[0158] The preparation of compound 2 of the present invention refers to Example 1, but replaces 2-chloromethylquinoline hydrochloride in Example 1 with 2-cyanobenzyl chloride, and prepares the compound corresponding to R in general formula (I). 1 2-cyanobenzyl xanthine.
[0159] A yellow solid was obtained. 1 H NMR (600MHz, DMSO-d 6 ):δ:7.87(d,J=7.8Hz,1H),7.85(d,J=7.2Hz,1H),7.67-7.62(m,2H),7.50-7.45(m,2H),7.39(d, J=7.6Hz,1H),7.29(d,J=8.0Hz,1H),5.32(s,2H),5.24(s,2H),4.97-4.91(m,2H),3.66-3.62(m,1H ),3.56-3.50(m,1H),3.15-3.09(m,2H),2.99-2.96(m,1H),1.93-1.89(m,1H),1.85-1.84(m,1H),1.81(brs ,3H),1.68-1.61(m,1H),1.50-1.43(m,1H).ESI-MS m / z:calc.532.2,found533.2[M+H] +
Embodiment 3
[0160] Example 3: Compound 3 (1,3-bis[(3-methoxycarbonyl)-benzyl]-7-(2-butyn-1-yl)-8-[(R)-3-amino- Preparation of piperidin-1-yl]-xanthine)
[0161] The preparation of compound 3 of the present invention refers to Example 1, but replaces 2-chloromethylquinoline hydrochloride in Example 1 with 3-methoxycarbonyl-benzyl chloride, and prepares the compound corresponding to R in general formula (I). 1 3-Methoxycarbonylbenzyl xanthine to give a yellow solid. 1 H NMR (600MHz, DMSO-d 6 ):δ:8.03-7.98(m,1H),7.89-7.86(m,2H),7.84(d,J=6.8Hz,1H),7.63(d,J=7.9Hz,1H),7.57(d, J=7.7Hz,1H),7.50-7.45(m,2H),5.18(s,2H),5.10(s,2H),4.97-4.90(m,2H),3.83(s,3H),3.82(s ,3H),3.68-3.65(m,1H),3.20-3.12(m,3H),2.01-1.96(m,1H),1.92-1.86(m,1H),1.80(brs,3H),1.71-1.65 (m,1H),1.63-1.58(m,1H).ESI-MS m / z:calc.598.2,found599.2[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com