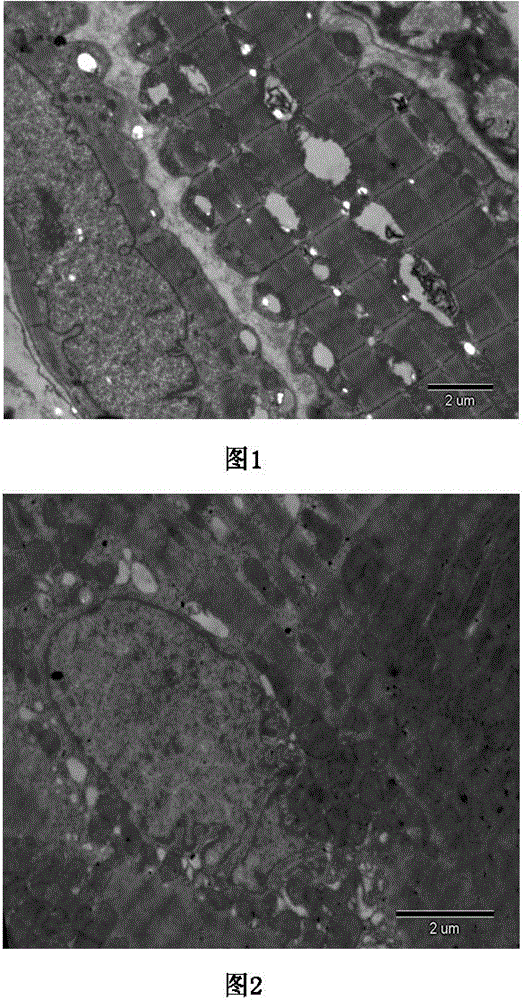
Autoimmune hemolytic anemia (ALHA) cardioplegic solution and preparation method thereof
A cardioplegia solution and solution technology, applied in the field of cardioplegia, can solve problems such as prone to arrhythmia, prone to inflammatory reactions, and long arrest time, so as to improve the success rate of a relapse and shorten the arrest Time, the effect of avoiding the damage of the myocardium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1, the ALHA cardioplegia solution contains 552.86mg to 947.76mg of adenosylcobalamin, 4.739ml to 5ml of lidocaine solution with a mass concentration of 2%, and pentazocine per 500ml of the ALHA cardioplegia solution. 30mg to 60mg, 4.1ml to 10ml of magnesium sulfate solution with mass concentration of 25%, 3.725ml to 5ml of potassium chloride solution with mass concentration of 10%, 4.3ml to 10ml of calcium gluconate solution with mass concentration of 10%, and the balance Acetate Ringer's solution; wherein, the ALHA cardioplegia solution is obtained by the following method: at a room temperature of 22°C to 26°C, oxygen is injected into the hyperoxic liquid at an oxygen flow rate of 2 L / min to 4L / min. In the instrument, the oxygen after the reaction is fed into the required amount of Ringer's acetate for dissolved oxygen activation treatment, and after dissolving oxygen, it is prepared into a hypertonic oxygen ringer's acetate whose partial pressure of oxygen in t...
Embodiment 2
[0022] Example 2, the ALHA cardioplegia solution contains 552.86 mg or 947.76 mg of adenosylcobalamin, 4.739 ml or 5 ml of lidocaine solution with a mass concentration of 2%, and pentazocine in every 500 ml of the ALHA cardioplegia solution. 30mg or 60mg, 4.1ml or 10ml of magnesium sulfate solution with mass concentration of 25%, 3.725ml or 5ml of potassium chloride solution with mass concentration of 10%, 4.3ml or 10ml of calcium gluconate solution with mass concentration of 10%, and the balance Acetate Ringer's solution; wherein, the ALHA cardioplegia solution is obtained by the following method: at a room temperature of 22 ° C or 26 ° C, oxygen is injected into the hyperoxic liquid at an oxygen flow rate of 2 L / min or 4 L / min. In the instrument, the oxygen after the reaction is fed into the required amount of Ringer's acetate for dissolved oxygen activation treatment, and after dissolving oxygen, it is prepared into hypertonic oxygen Ringer's acetate with an oxygen partial p...
Embodiment 3
[0023] Embodiment 3, the preparation method of this ALHA cardioplegia solution, every 500ml of this ALHA cardioplegia solution contains adenosylcobalamin 552.86mg to 947.76mg, mass concentration is 2% lidocaine solution 4.739ml to 5ml, spray Tazocine 30mg to 60mg, 25% magnesium sulfate solution 4.1ml to 10ml, 10% potassium chloride solution 3.725ml to 5ml, 10% calcium gluconate solution 4.3ml to 10ml and the remaining amount of Ringer's acetate solution; wherein, the preparation method of the ALHA cardioplegia solution is carried out according to the following steps: at a room temperature of 22°C to 26°C, with an oxygen flow rate of 2 L / min to 4L / min Input oxygen into the hyperoxic liquid preparation apparatus, and pass the reacted oxygen into the required amount of acetic acid Ringer's solution for oxygen-dissolving activation treatment. After dissolving oxygen, prepare a hypertonic liquid with an oxygen partial pressure of 100kPa to 120kPa in the solution. Oxygenated Ringer'...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com