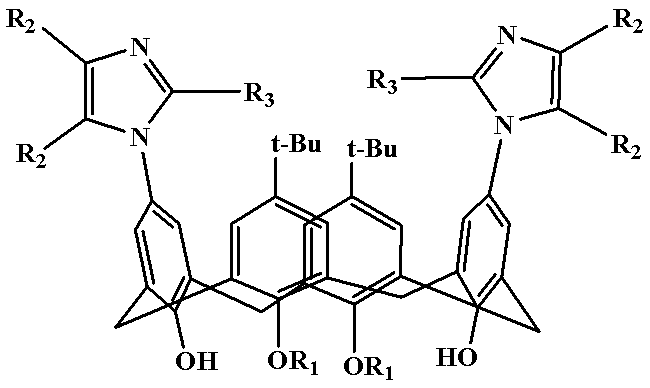
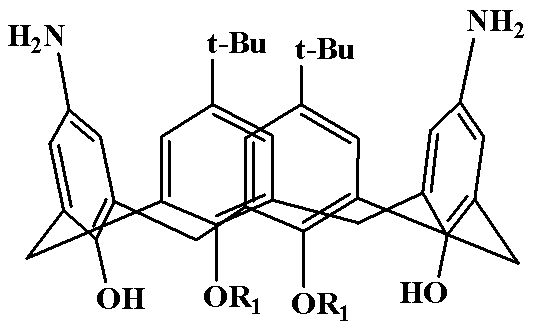
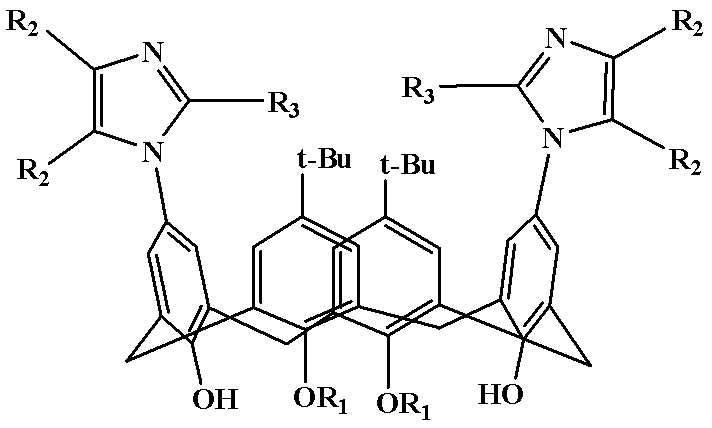
Polysubstitution imidazole calixarene derivative and preparation method thereof
A technology of arene derivatives and derivatives, applied in organic chemistry and other fields, can solve problems that cannot satisfy researchers' research on the application performance of calixarene derivatives, and achieve the effect of novel structure, fewer steps, and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021] Example 1: Preparation of Polysubstituted Imidazocalixarene Derivative 1
[0022] (5,17-Di-tert-butyl-11,23-bis(2,4,5-triphenylimidazolyl)-25,27-dibutoxycalix[4]arene)
[0023] Diaminocalixarene derivative (5.0 mmol), benzil (7.5 mmol), benzaldehyde (5.0 mmol) and ammonium acetate (5.0 mmol) were added to 100 mL of acetic acid, and the temperature was slowly raised to 120 °C for 10 hours. After the reaction is completed, add about ice water while still hot, stir rapidly, use 5% NaOH solution to adjust the pH value of the reaction system between 7 and 8, and precipitate a large amount of solid; after filtration, the solid is dissolved in dichloromethane, and saturated brine is used. The organic phase was washed 2-3 times and dried over anhydrous magnesium sulfate overnight. The drying agent was filtered off and the organic phase was concentrated to give the crude product as a dark red oil. The crude product was recrystallized from chloroform to obtain 5,17-di-tert-buty...
example 2
[0024] Example 2: Preparation of polysubstituted imidazole calixarene derivatives 2
[0025] (5,17-Di-tert-butyl-11,23-bis(4,5-diphenyl-2-p-chlorophenylimidazolyl)-25,27-dibutoxycalix[4]arene)
[0026] Diaminocalixarene derivative (5.0 mmol), benzil (8.0 mmol), p-chlorobenzaldehyde (6.0 mmol) and ammonium acetate (7.5 mmol) were added to the reaction 100 mL of formic acid, and the temperature was slowly raised to 100 °C for reaction 12 Hour. After the reaction is completed, add about ice water while still hot, stir rapidly, use 5% NaOH solution to adjust the pH value of the reaction system between 7 and 8, and precipitate a large amount of solid; after filtration, the solid is dissolved in dichloromethane, and saturated brine is used. The organic phase was washed 2-3 times and dried over anhydrous magnesium sulfate overnight. The drying agent was filtered off and the organic phase was concentrated to give the crude product as a dark red oil. The crude product was recrystall...
example 3
[0027] Example 3: Preparation of multiple substituted imidazolium calixarene derivatives 3
[0028] (5,17-di-tert-butyl-11,23-bis(4,5-diphenyl-2-p-methoxyphenylimidazolyl)-25,27-dibutoxycalix[4]arene)
[0029] Diaminocalixarene derivative (5.0 mmol), benzil (10.0 mmol), p-methoxybenzaldehyde (10.0 mmol) and ammonium acetate (10.0 mmol) were added to the reaction 100 mL of isopropanol, and the temperature was slowly raised to 80 °C, react for 12 hours. After the reaction is over, add about ice water while it is hot, stir quickly, adjust the pH value of the reaction system between 7 and 8 with 5% NaOH solution, and precipitate a large amount of solids; The organic phase was washed 2-3 times, and dried overnight with anhydrous magnesium sulfate. The desiccant was filtered off, and the organic phase was concentrated to obtain a dark red oily crude product. The crude product was recrystallized from acetonitrile to obtain 5,17-di-tert-butyl-11,23-bis(4,5-diphenyl-2-p-methoxypheny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com