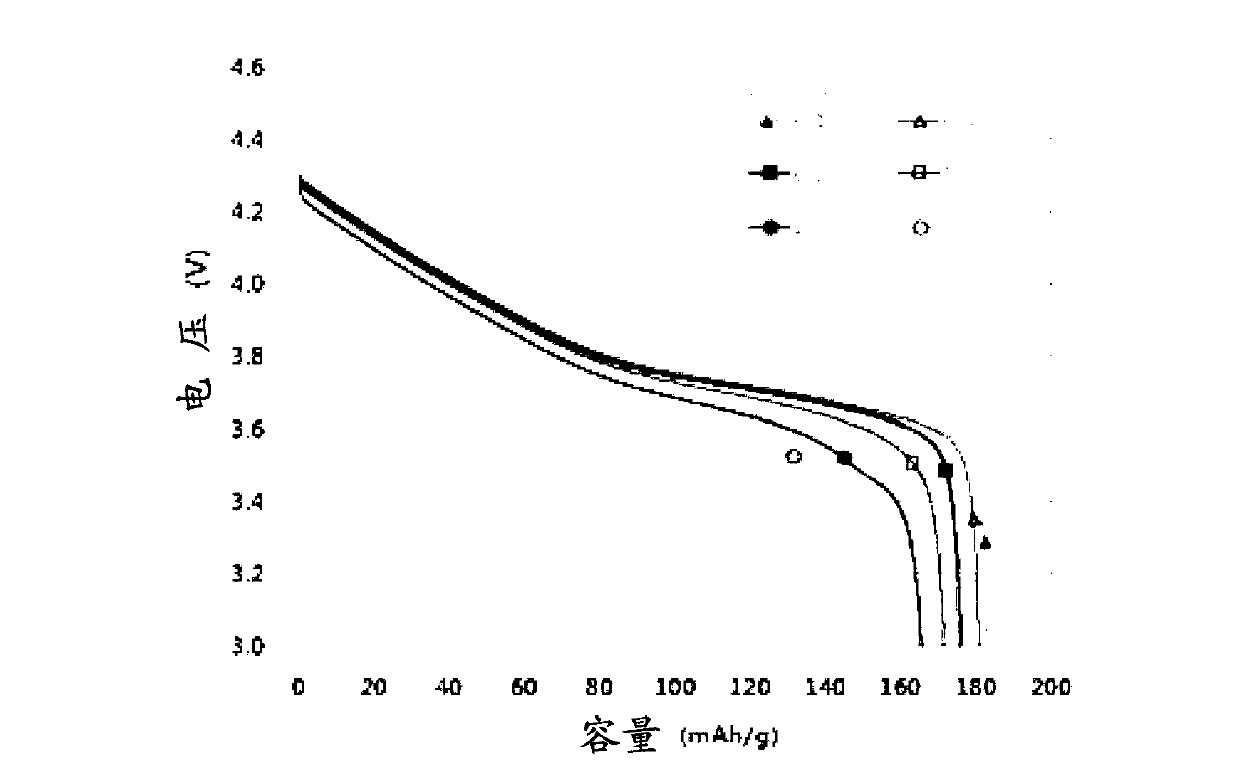
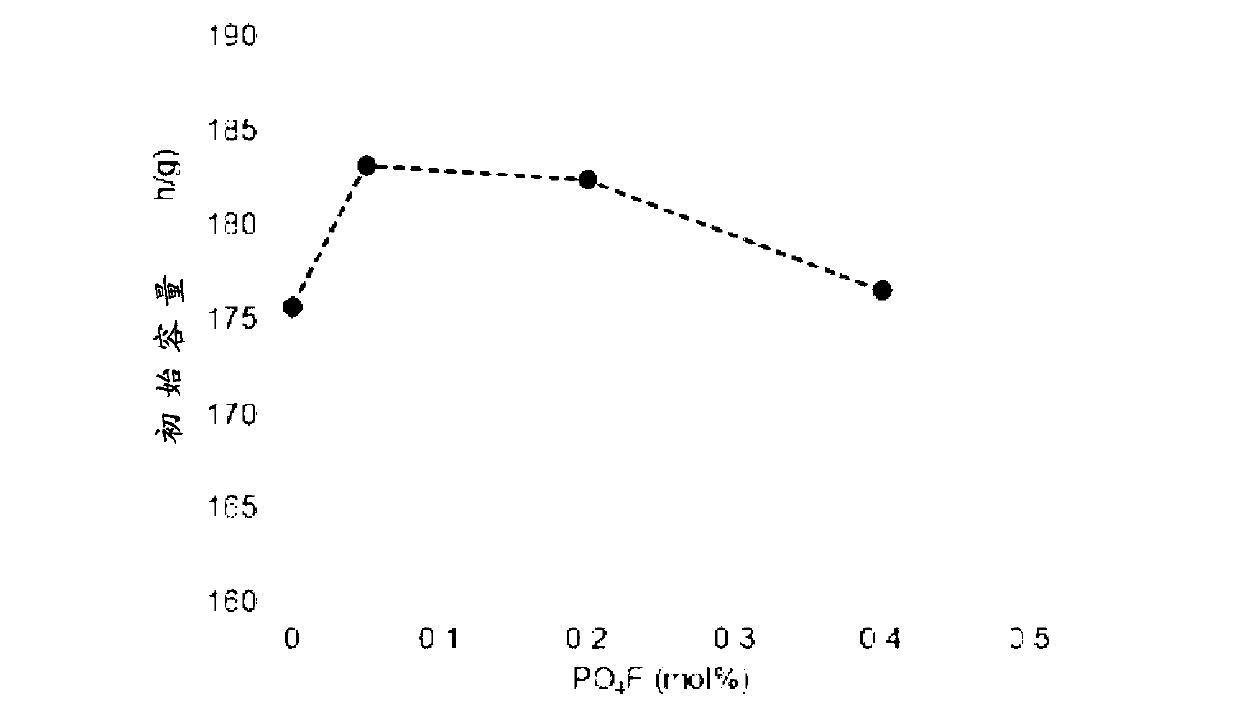
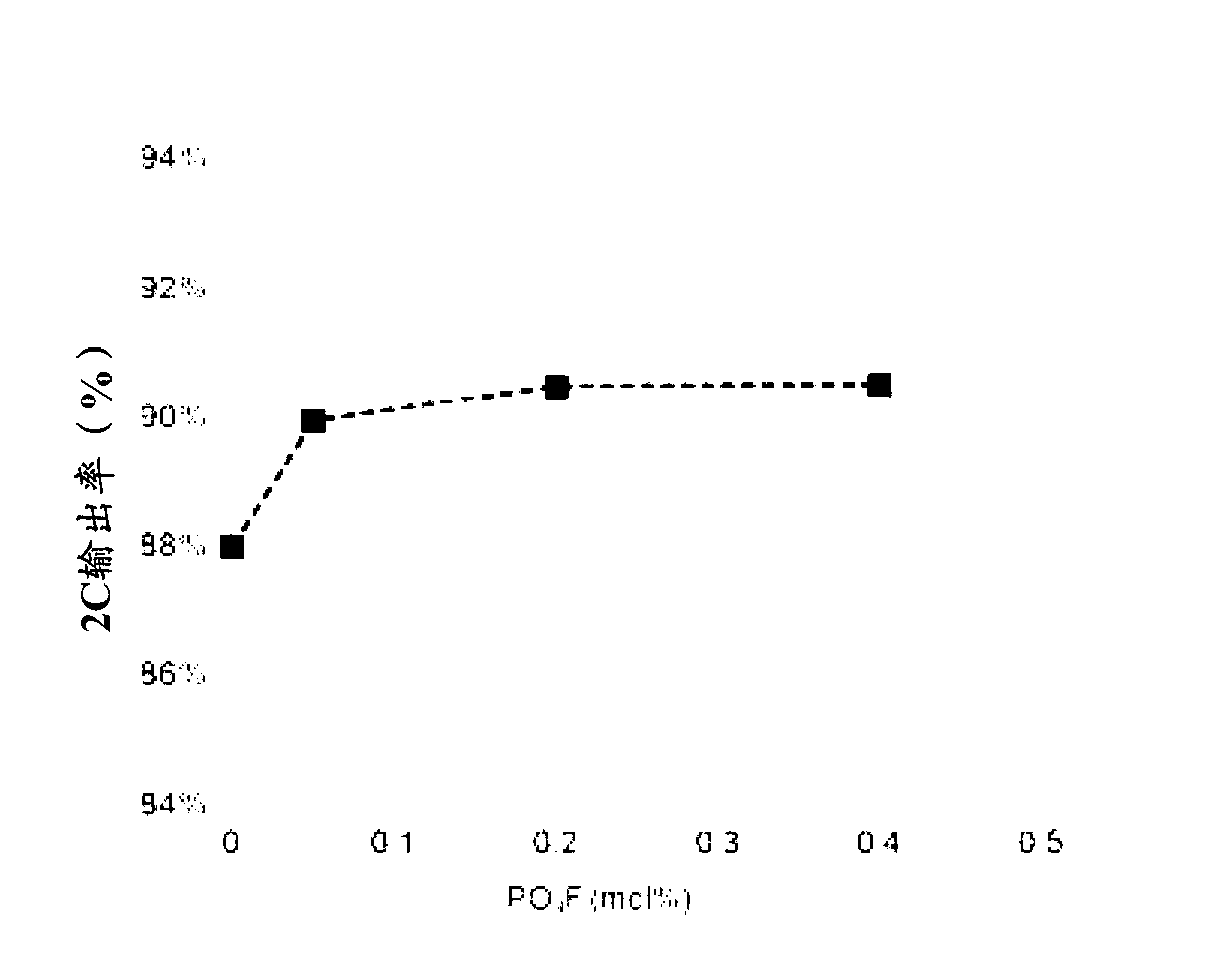
Cathode active material for a lithium secondary battery containing phosphate fluoride and preparation method thereof
A cathode active material, lithium secondary battery technology, applied in secondary batteries, battery electrodes, lithium storage batteries, etc., can solve the problems of limited stability improvement effect, low energy density, limitations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] In one embodiment, the preparation method of the cathode active material of the present invention comprises the steps of: (a) combining a precursor containing nickel, cobalt and manganese with a lithium-containing compound, a fluorine (F)-containing compound, and a phosphate-containing (PO4 ) together; and (b) sintering the mixture obtained in step (a) to obtain the compound of formula 1 above further doped or coated with fluorophosphate.
[0040] The fluorine-containing compound can be selected from NH 4 F, NH 4 HF 2 , NH 4 PF 6 , LiF, LiAlF 6 , AlF 3 , MgF 2 , CaF 2 , MnF 2 , MnF 3 , FeF 2 , FeF 3 、CoF 2 、CoF 3 、NiF 2 、TiF 4 , CuF, CuF 2 , ZnF 2 , polyvinylidene fluoride, polyvinylidene fluoride-hexafluoropropylene copolymer, and a group consisting of mixtures thereof. The phosphate-containing compound can be selected from NH 4 h 2 PO 4 , (NH 4 ) 2 HPO 4 、H 3 PO 4 , Li 3 PO 4 , LiH 2 PO 4 , MgHPO 4 , Mg 3 (PO 4 ) 2 , Mg(H 2 PO 4 ) ...
preparation example 1
[0058] Preparation Example 1: Preparation of cathode active material precursor (Ni:Co:Mn=6:2:2)
[0059] NiSO 4 ·6H 2 O. CoSO 4 ·7H 2 O and MnSO 4 ·H 2 O was mixed in a molar ratio of 6:2:2, and 2 Purified distilled water to prepare a 2M metal salt solution. The metal salt solution was poured into a continuous stirred tank reactor (CSTR) at a flow rate of 250 mL / hr.
[0060] A 25% ammonia solution was poured into the reactor through the ammonia solution inlet at a flow rate of 40 mL / hr. Further, 40% NaOH aqueous solution was automatically poured into the reactor through the NaOH solution inlet at a flow rate of 105-120 mL / hr, and at the same time, the pH was maintained at 11.3 by using a pH measuring instrument and a regulator. The temperature of the reactor was set at 40° C., the holding time (RT) was controlled at 10 hr, and the mixed solution was stirred at 800 rpm.
[0061] The resulting reaction mixture was filtered, purified with distilled water, and dried to ob...
preparation example 2
[0062] Preparation Example 2: Preparation of cathode active material precursor (Ni:Co:Mn=7:1:2)
[0063] NiSO 4 ·6H 2 O. CoSO 4 ·7H 2 O and MnSO 4 ·H 2 O was mixed in a molar ratio of 7:1:2, and 2 Purified distilled water to prepare a 2M metal salt solution. The metal salt solution was poured into a 1 L capacity Couette-Taylor reactor through the metal salt solution inlet at a flow rate of 200 mL / hr.
[0064] A 25% ammonia solution was poured into the reactor through the ammonia solution inlet at a flow rate of 35 mL / hr. Further, 40% NaOH aqueous solution was automatically poured into the reactor through the NaOH solution inlet at a flow rate of 85-100 mL / hr, and at the same time, the pH was maintained at 11.2 using a pH measuring instrument and a regulator. The temperature of the reactor was set at 40° C., the holding time (RT) was controlled at 3 hr, and the mixed solution was stirred at 600 rpm.
[0065] The resulting reaction mixture was filtered, purified with di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com