Glucagon analogues
A compound and residue technology, applied in glucagon, hormone peptides, drug combinations, etc., can solve the problem that the mechanism of action of oxyntomodulin is not very clear.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0387] The peptide components of the compounds of the invention may be prepared by standard synthetic methods, by using recombinant expression systems, or any other suitable method. Thus, the peptides can be synthesized in a variety of ways including, for example, methods comprising:
[0388] (a) peptide synthesis by stepwise synthesis or by fragment assembly, using solid or liquid phase methods, and isolation and purification of the final peptide product; or
[0389] (b) expressing the nucleic acid construct encoding the peptide in a host cell and recovering the expression product from the host cell culture; or
[0390](c) performing cell-free in vitro expression of the nucleic acid construct encoding the peptide, and recovering the expression product;
[0391] Or use any combination of methods (a), (b) and (c) to obtain peptide fragments, which are subsequently ligated to obtain complete peptides, and the peptides are recovered.
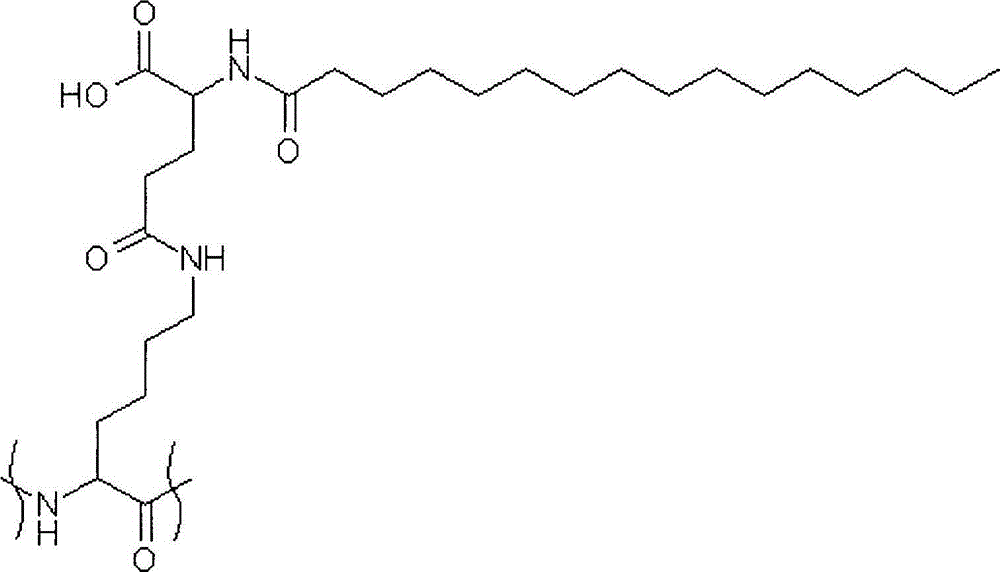
[0392] Compounds of the invention may ofte...
Embodiment 1
[0482] Example 1: Efficacy on GLP-1 and Glucagon Receptors
[0483] The potency of the compounds of the invention was assessed by exposing cells expressing human glucagon-R and human GLP-1R to increasing concentrations of the compounds listed in Table 1 below and measuring the cAMP formed as described in the Methods section above .
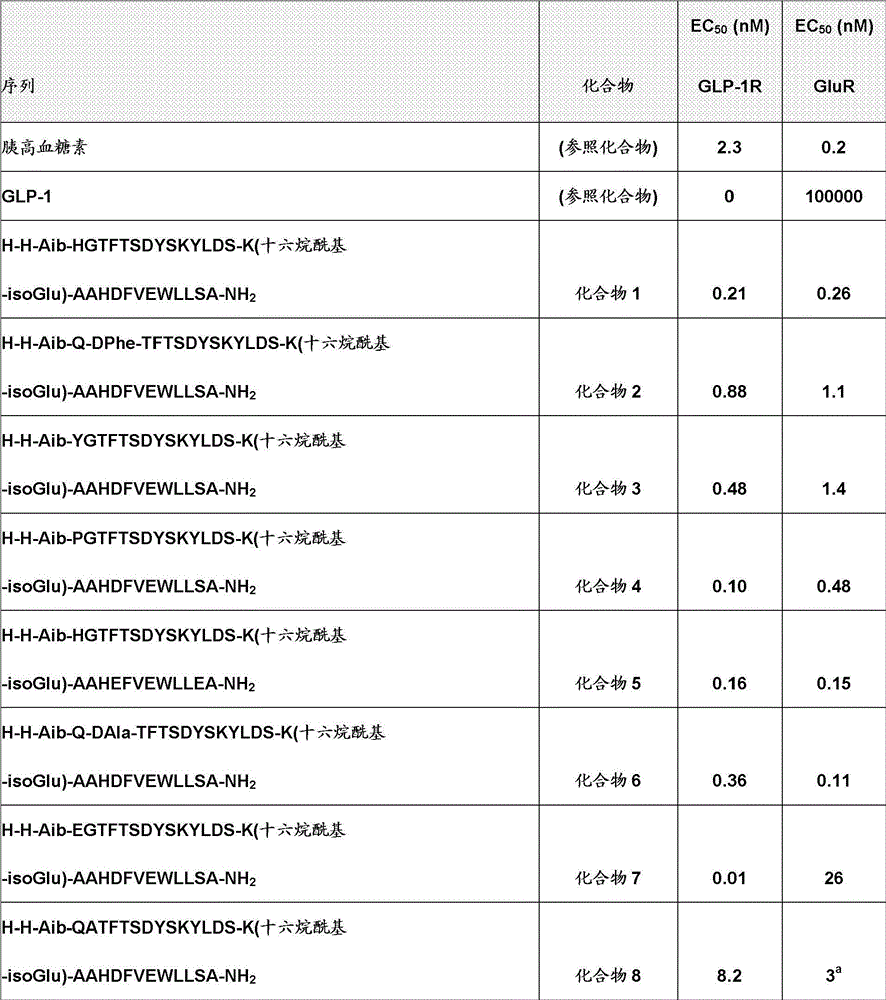
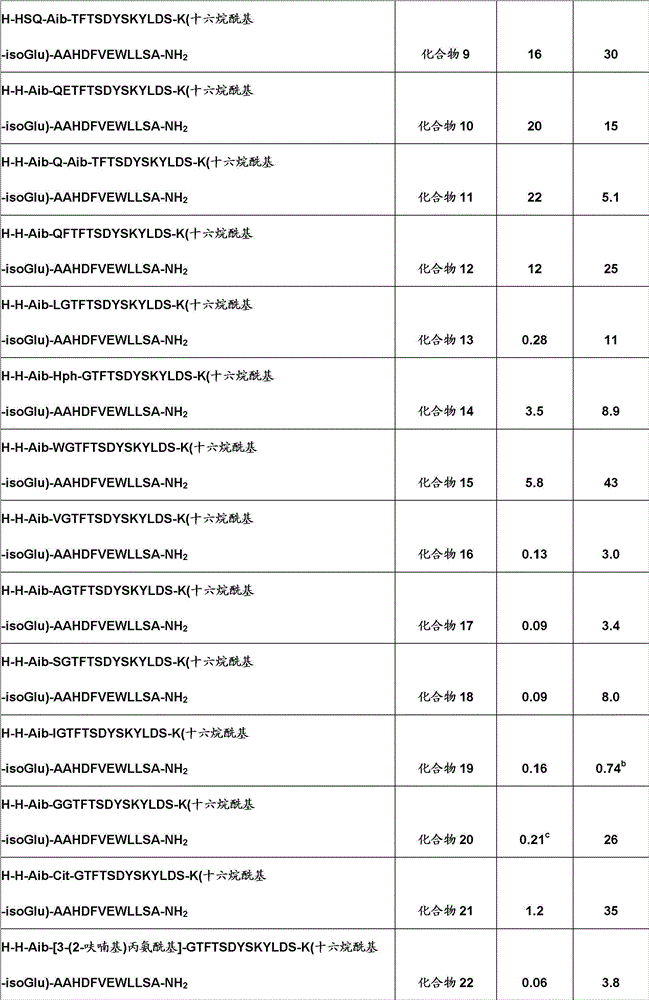
[0484] The EC of the compound of the present invention is shown in table 1 50 Results, and corresponding values for human glucagon and human GLP-1 as reference compounds:
[0485] Table 1. Effects of compounds of the invention on GLP-1 and glucagon receptors (EC 50 )
[0486]
[0487]
[0488]
[0489] a Regarding the re-measurement relative to the earlier determination value of 23.
[0490] b Re-determination relative to an earlier determination of 3.0.
[0491] c Re-assay relative to earlier determination of 0.89.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com