One-pot preparation method of vitamin B6
A vitamin and methyl technology, applied in the field of medicine and biochemical industry, can solve the problems of difficult decolorization, high price, heavy product coloring, etc., and achieve the effects of short process flow, high selectivity and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
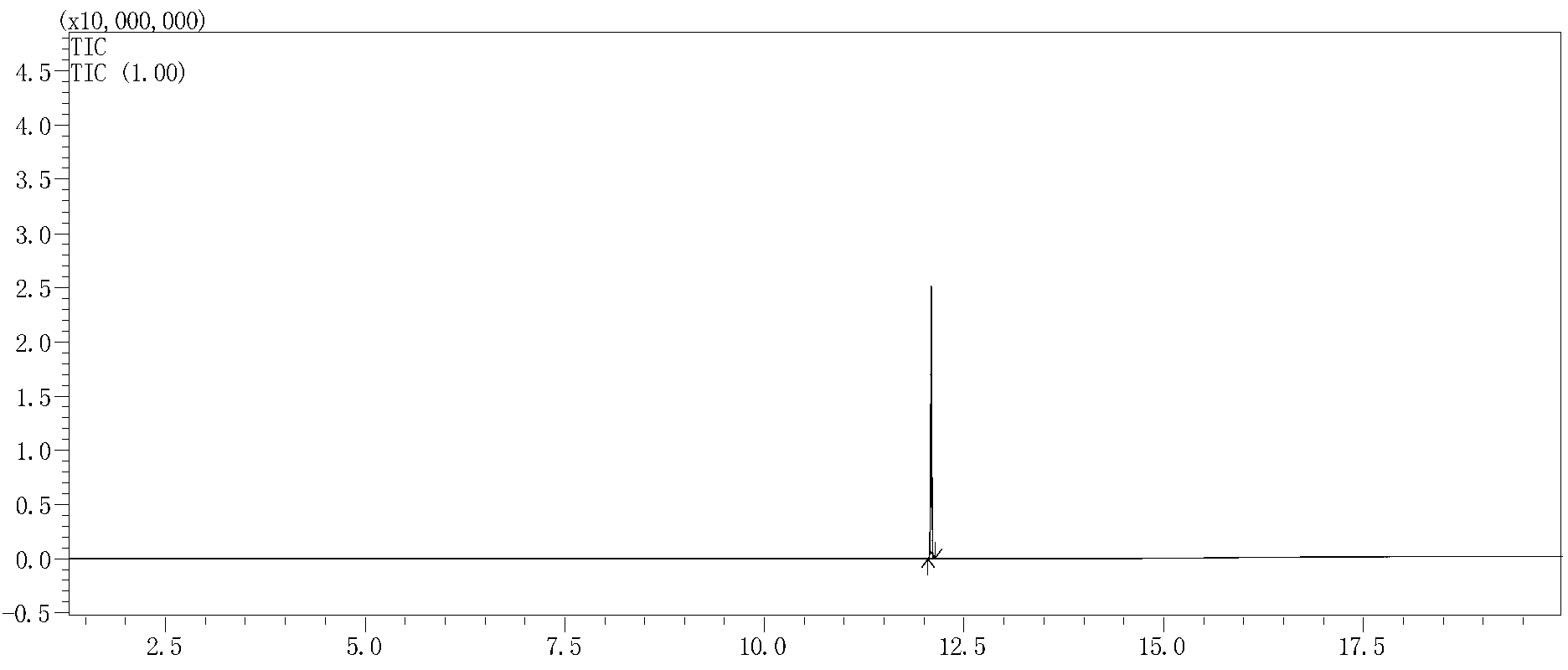
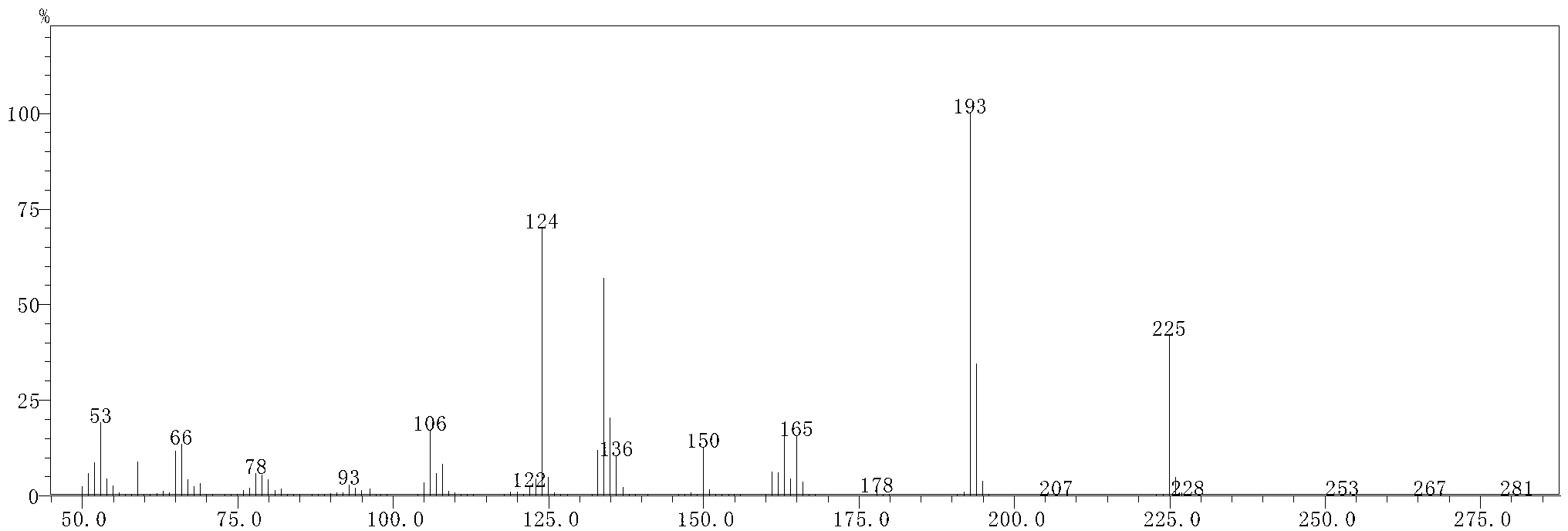
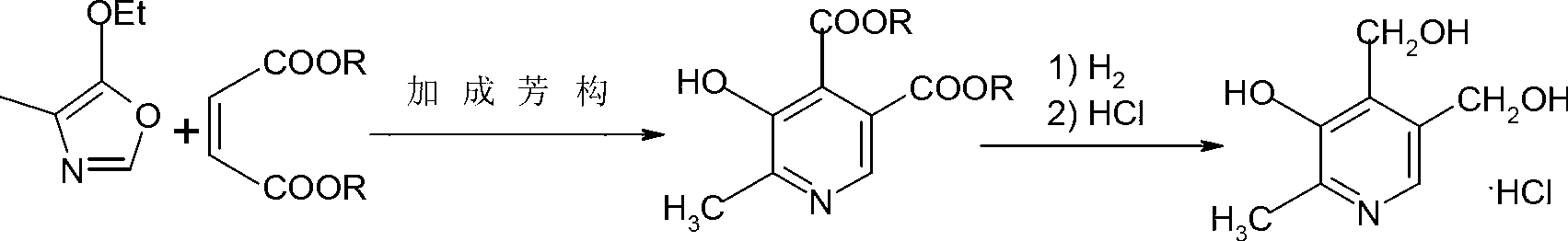
[0034] Add 144g of dimethyl maleate and 127g of 4-methyl-5-ethoxy oxazole to a dry 500mL four-neck flask, heat up to 80°C and react for 15 hours to obtain 2-methyl-3-hydroxy-pyridine Dimethyl-4,5-dicarboxylate 221.5g, purity 98.5% (GC), yield 97%, the product was confirmed by GC-MS analysis, see figure 1 and figure 2 .
[0035] Put 56g of 2-methyl-3-hydroxy-pyridine-4,5-dicarboxylate into a 500mL autoclave, 202g of methanol as a solvent, 6g of activated T-1 Raney nickel catalyst, nitrogen replacement three times, Replace with hydrogen three times, then add hydrogen for reduction, continue to flow hydrogen during the reaction process, keep the reaction pressure 2.0MPa, temperature 65°C, hydrogenation reaction for 6 hours, lower the temperature, filter to remove the catalyst; pass hydrogen chloride gas until saturated to form a salt, and recover methanol under reduced pressure. Then add 65mL of ethanol, take a water bath at 70°C, hold time for 30 minutes, slowly cool down to ...
Embodiment 2
[0037] Add 144g of dimethyl maleate and 127g of 4-methyl-5-ethoxy oxazole to a dry 500mL four-neck flask, heat up to 90°C and react for 13 hours to obtain 2-methyl-3-hydroxy-pyridine Dimethyl-4,5-dicarboxylate 219g, purity 98.2% (GC), yield 95.6%.
[0038]Put 56g of 2-methyl-3-hydroxy-pyridine-4,5-dicarboxylate into a 500mL autoclave, 202g of methanol as solvent, 8g of activated T-1 type Raney nickel catalyst, nitrogen replacement three times, The hydrogen was replaced three times, and then hydrogenated for reduction. During the reaction process, the hydrogen gas was continuously passed, and the reaction pressure was kept at 2.5Mpa, and the temperature was 68°C. The hydrogenation reaction was carried out for 5 hours, and the temperature was lowered. Then add 65mL of ethanol, take a water bath at 70°C, keep time for 30min, then slowly cool down to 15-20°C, and filter with suction for 30min to crystallize. 90.1%.
Embodiment 3
[0040] Add 158g of dimethyl maleate and 127g of 4-methyl-5-ethoxy oxazole to a dry 500mL four-neck flask, heat up to 100°C and react for 11 hours to obtain 2-methyl-3-hydroxy-pyridine Dimethyl-4,5-dicarboxylate 222g, purity 98.9% (GC), yield 97.6%.
[0041] Put 56g of 2-methyl-3-hydroxy-pyridine-4,5-dicarboxylate into a 500mL autoclave, 202g of methanol as solvent, 10g of activated T-1 type Raney nickel catalyst, nitrogen replacement three times, The hydrogen was replaced three times, and then hydrogenated for reduction. During the reaction process, the hydrogen was continuously passed, and the reaction pressure was kept at 3.0Mpa, and the temperature was 68°C. The hydrogenation reaction was carried out for 5 hours, and the temperature was lowered. Then add 65mL of ethanol, take a water bath at 70°C, keep timed for 30 minutes, then slowly cool down to 15-20°C, and filter with suction for 30 minutes to crystallize. The rate is 91.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com