A compound tablet with a high content of chondroitin sulfate and a preparation method thereof
A chondroitin sulfate and composite tablet technology is applied in food preparation, medical preparations containing active ingredients, pharmaceutical formulations, etc., and can solve the problems of easy pockmarking of the coating and difficulty in disintegrating chondroitin sulfate swallowed tablets, etc. To achieve the effect of being beneficial to carboxylation, promoting bone mineralization rate and bone formation rate, and preventing and treating osteoporosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
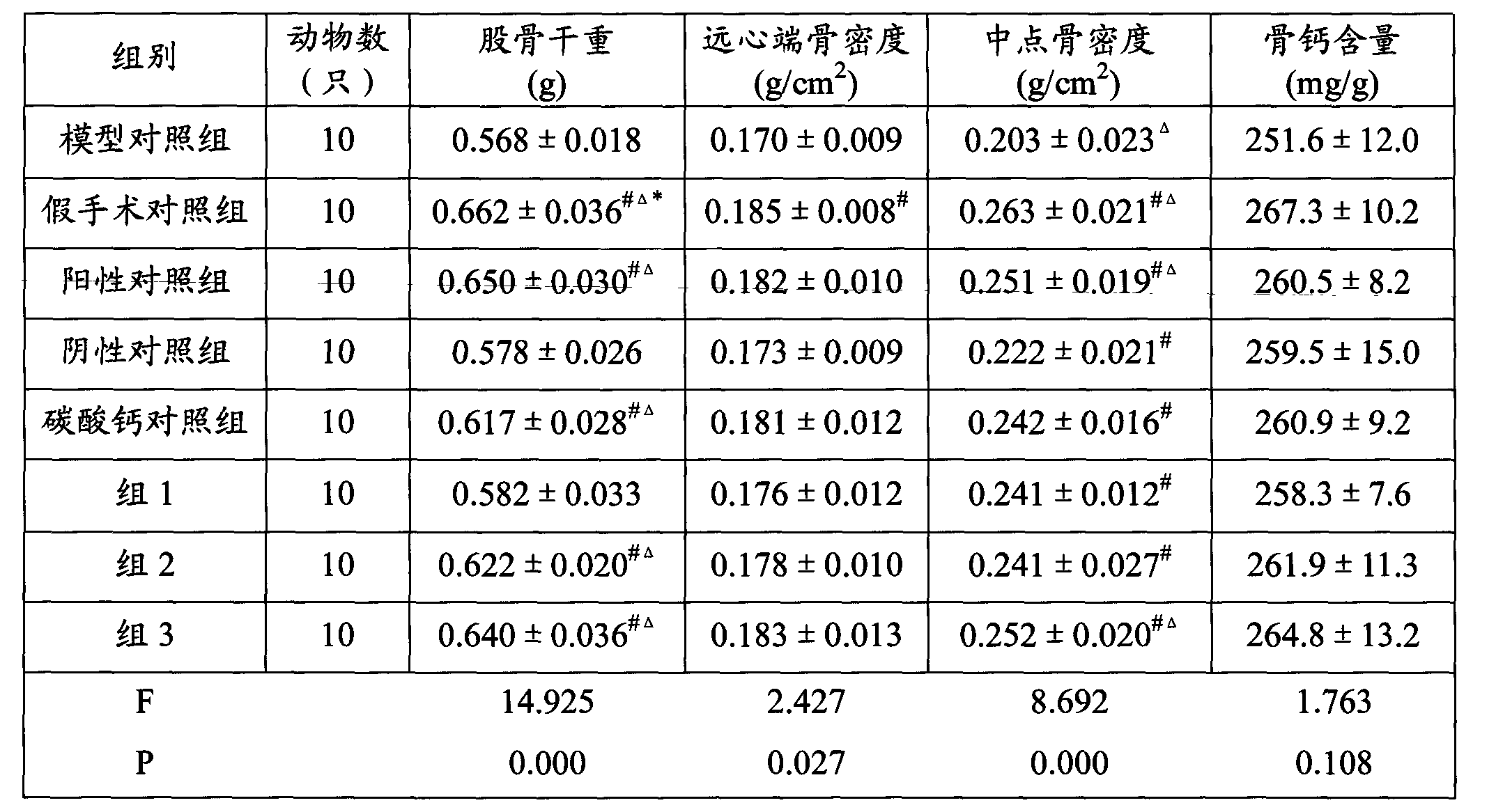
Examples
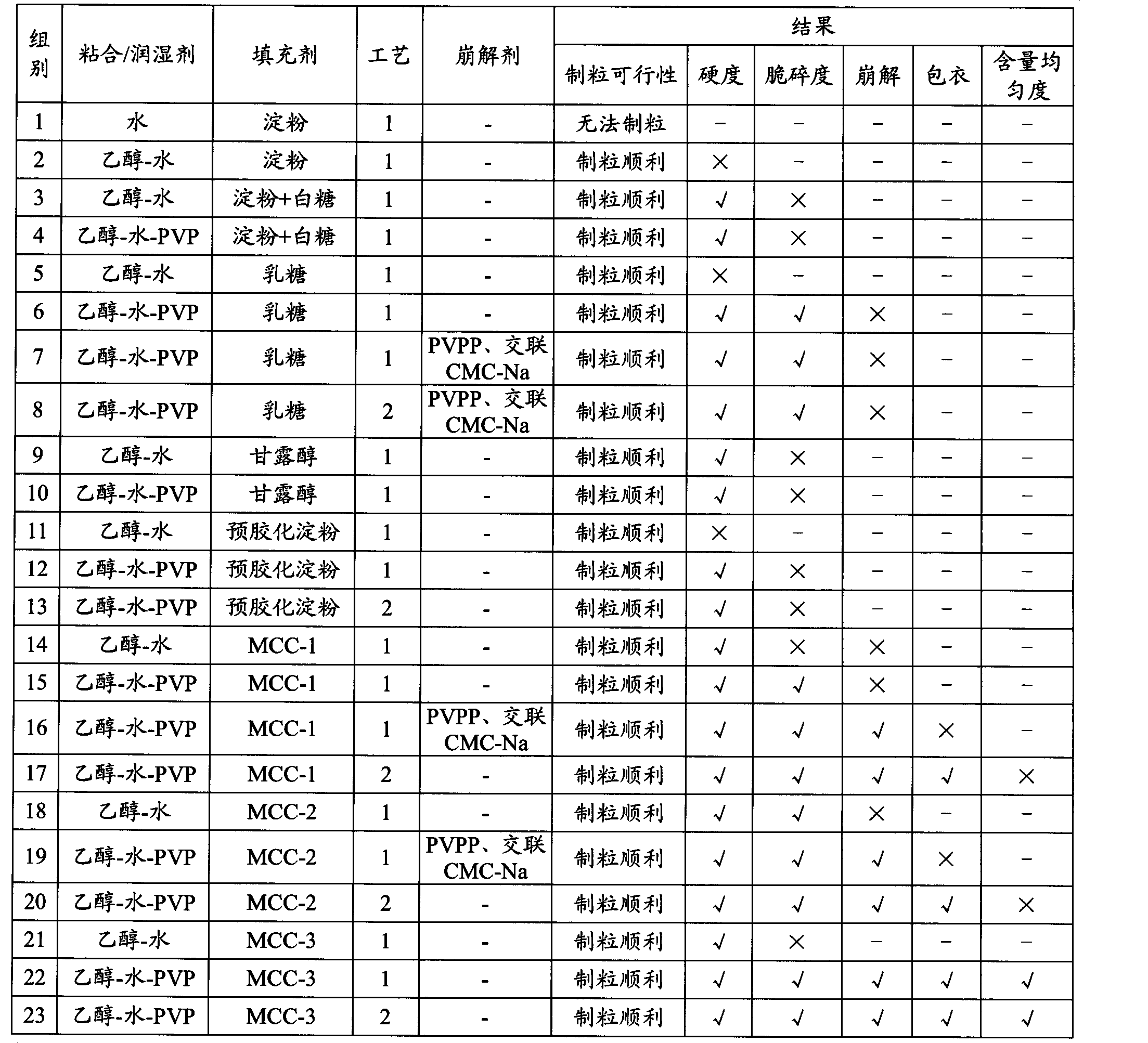
Embodiment 1
[0044] (1) Screening of raw materials: 27% chondroitin sulfate, 28% glucosamine, 12% calcium phosphate, and 0.2% casein phosphopeptide were respectively passed through a 100-mesh sieve accounting for tablet mass ratio.
[0045] (2) Chondroitin sulfate granulation:
[0046] a. Add 0.8% povidone solution to the chondroitin sulfate treated in (1) to make a soft material.
[0047] b. Wet granules: Pass the chondroitin sulfate soft material prepared in (2)a through a 20-mesh sieve to make wet granules.
[0048] c. Drying: Dry the chondroitin sulfate wet granules prepared in (2) b at a temperature of 60° C. for 60-100 min until the water content of the granules is 2-4%.
[0049] d. Grain sizing: pass the dry chondroitin sulfate granules prepared in (2) c through a 40-mesh sieve. spare.
[0050] (3) Glucosamine, calcium phosphate, casein phosphopeptide, vitamin K 2 Granulation:
[0051] a, making soft material: fully mix (1) treated glucosamine, calcium phosphate, casein phospho...
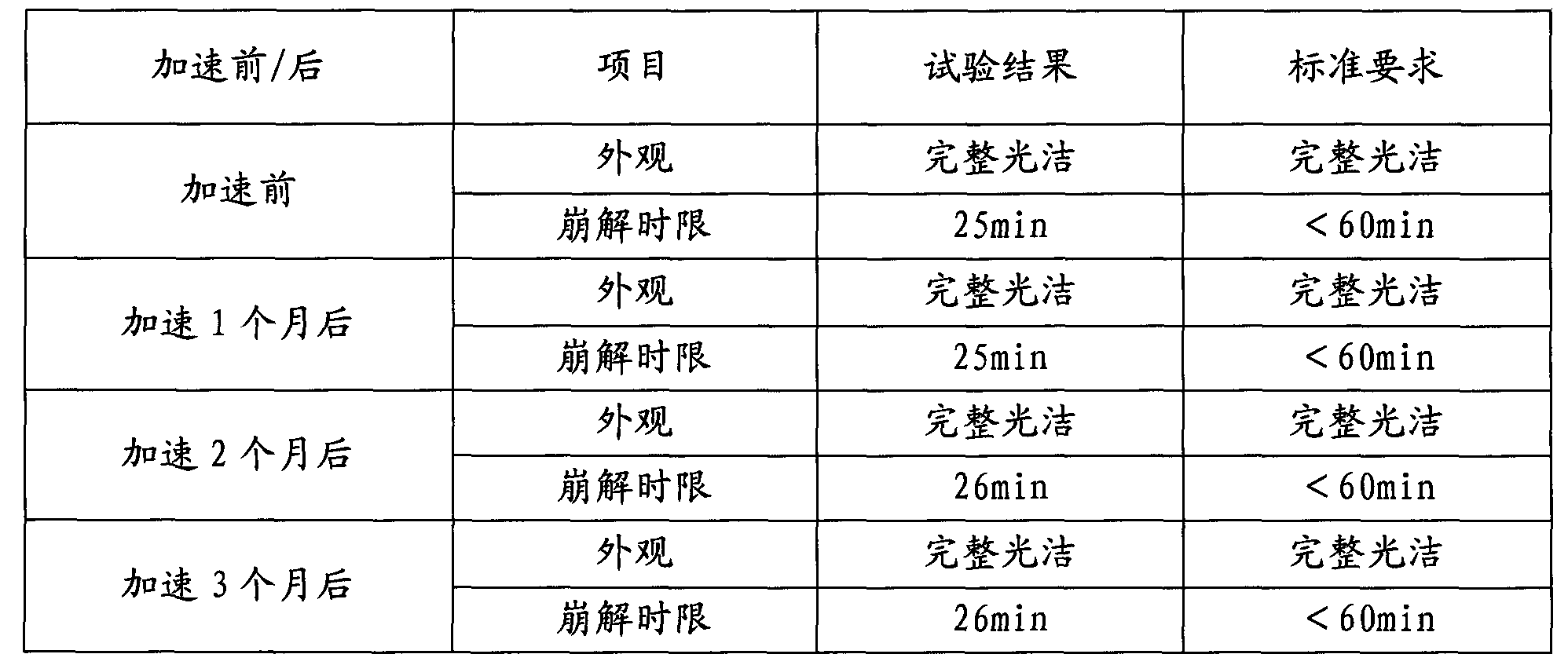
Embodiment 2
[0058] (1) Screening of raw materials: 32% chondroitin sulfate, 25% glucosamine, 15% calcium phosphate and 0.4% casein phosphopeptide were passed through an 80-mesh sieve accounting for tablet mass ratio.
[0059] (2) soft material: the processed chondroitin sulfate, glucosamine, calcium phosphate, casein phosphopeptide and 0.6% vitamin K 2 A soft material is made, and the binder is a povidone solution, and the povidone accounts for 1.5% of the sheet weight.
[0060] (3) Humidification granules: pass the soft material prepared in (2) through a 20-mesh sieve to make granules.
[0061] (4) Drying: Dry the wet granules prepared in (3) at a temperature of 50-70° C. for 20-120 minutes until the water content of the granules is 2-4%.
[0062] (5) Grain sizing: Pass the dry granules prepared in (4) through a 40-mesh sieve. spare.
[0063] (6) Blending: Add 21% microcrystalline cellulose and 0.5% magnesium stearate to the dry granules after (5) granulation for blending.
[0064] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com