5-bromo oxoisoaporphine, and synthesis method and application thereof
A technique for synthesizing 5-bromooxyisoaporphine, which is applied in the field of medicine, can solve problems such as the synthesis method and application of 5-bromooxyisoaporphine, and achieve significant in vitro antitumor activity and good medicinal value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
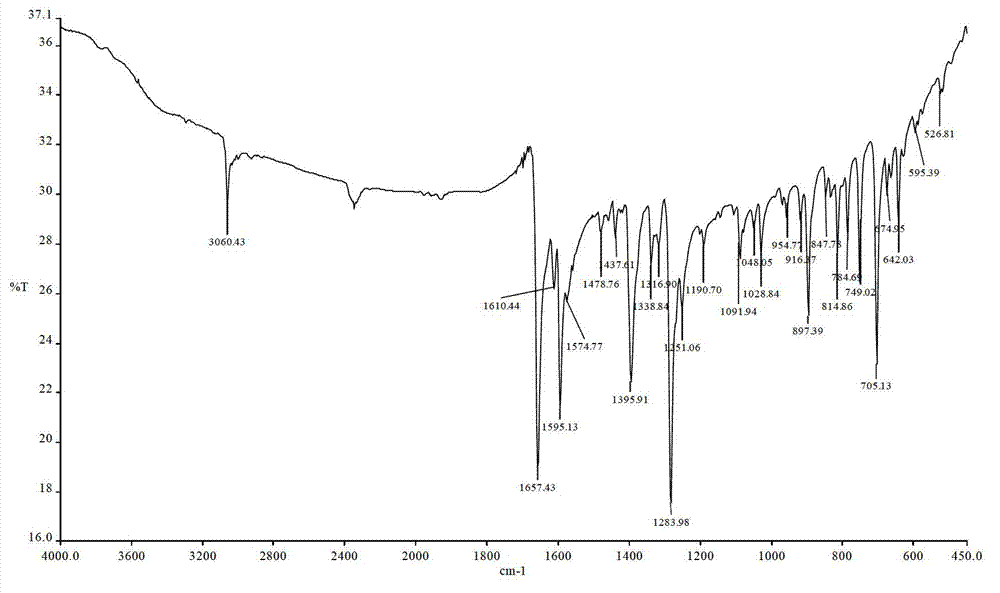
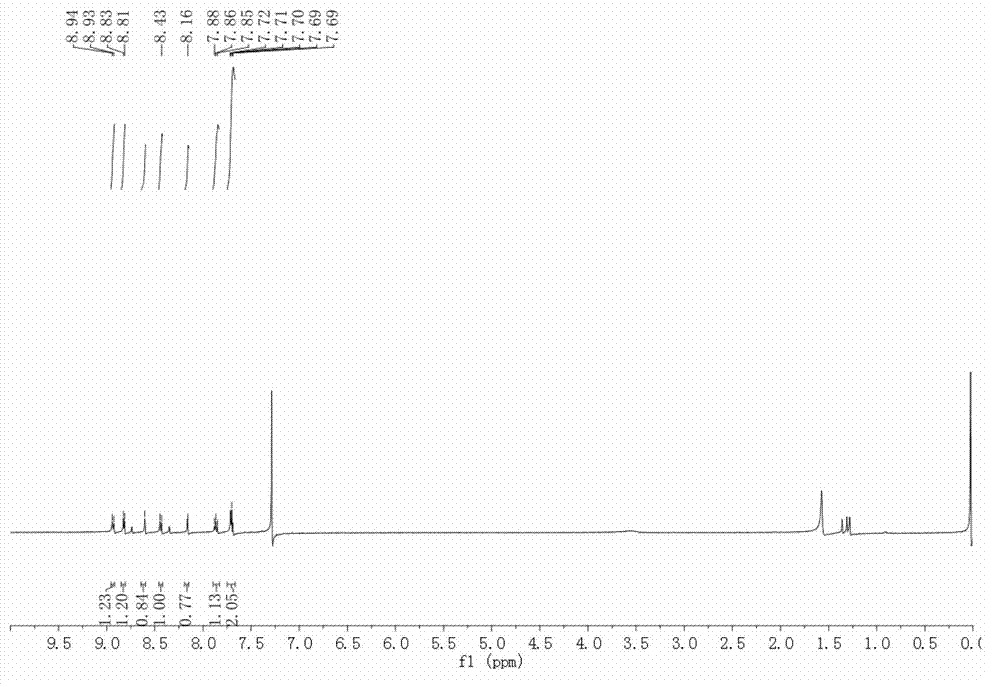
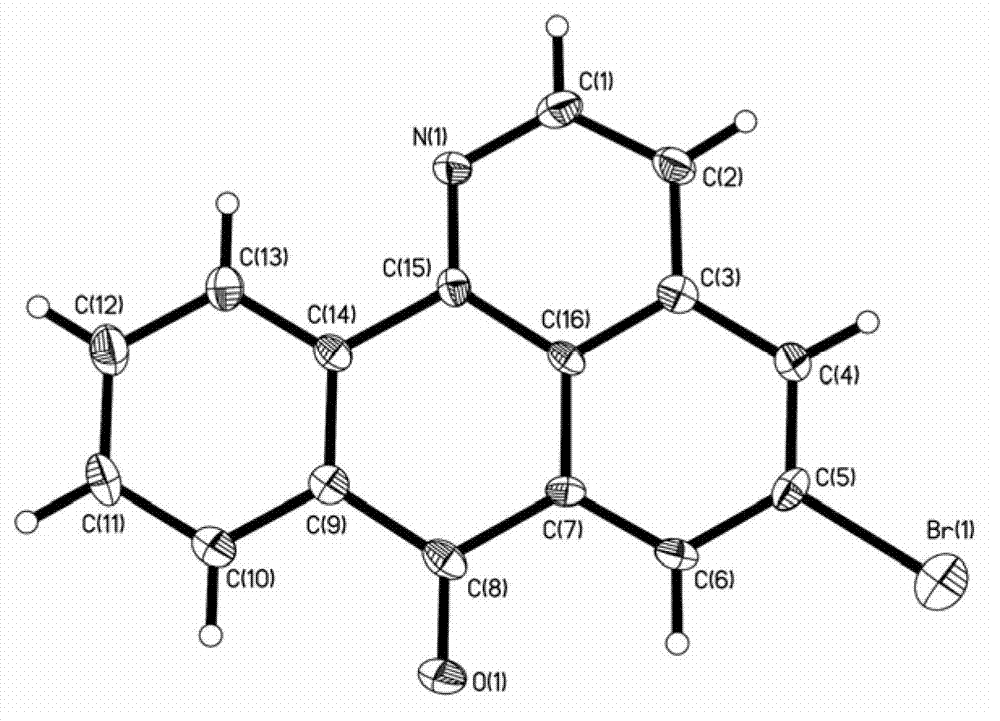
Image
Examples
Embodiment 1
[0035] 1) Synthesis of intermediate 1 (4-bromophenethylamine):
[0036] Dissolve 0.2mol of lithium aluminum hydride in 200mL of anhydrous ether, and dissolve 0.2mol of 4-bromophenylacetonitrile in 50mL of anhydrous ether. Slowly add the anhydrous ether solution of 4-bromophenylacetonitrile dropwise. After the addition is complete, stir and react at room temperature for 6 hours, neutralize with saturated sodium sulfate solution until no bubbles are generated, filter the reaction solution with suction, wash the filter residue with chloroform, and depressurize The solvent was distilled off to obtain intermediate 1 (colorless and slightly viscous 4-bromophenethylamine liquid), with a yield of 40%;
[0037] 2) Synthesis of Intermediate 2:
[0038] Mix and dissolve 0.1 mol of phthalic anhydride and 0.1 mol of intermediate 1 in 200 mL of ethanol, reflux at 90°C for 8 hours, cool the reaction solution to room temperature, and precipitate colorless crystals, filter them with suction, ...
Embodiment 2
[0050] 1) Synthesis of intermediate 1 (4-bromophenethylamine):
[0051] Dissolve 0.1 mol of lithium aluminum hydride in 100 mL of anhydrous ether, and dissolve 0.2 mol of 4-bromophenylacetonitrile in 50 mL of anhydrous ether. Slowly add the anhydrous ether solution of 4-bromophenylacetonitrile dropwise. After the addition is complete, stir and react at room temperature for 8 hours, neutralize with saturated sodium sulfate solution until no bubbles are generated, filter the reaction solution with suction, wash the filtrate with chloroform, and depressurize The solvent was distilled off to obtain intermediate 1 (colorless and slightly viscous 4-bromophenethylamine liquid), with a yield of 20%;
[0052] 2) Synthesis of Intermediate 2:
[0053] Take 0.1mol of phthalic anhydride and 0.1mol of intermediate 1, mix and dissolve in 50mL of ethanol, reflux at 95°C for 2 hours, suction filter while it is hot, cool the filtrate to room temperature, and precipitate colorless crystals, suc...
Embodiment 3
[0059] 1) Synthesis of intermediate 1 (4-bromophenethylamine):
[0060] Dissolve 0.4mol of lithium aluminum hydride in 300mL of anhydrous ether, and dissolve 0.2mol of 4-bromophenylacetonitrile in 50mL of anhydrous ether. Slowly add the anhydrous ether solution of 4-bromophenylacetonitrile dropwise. After the addition is complete, stir the reaction at room temperature for 2 hours, neutralize with saturated sodium sulfate solution until no bubbles are generated, filter the reaction solution with suction, wash the filtrate with chloroform, and depressurize The solvent was distilled off to obtain intermediate 1 (colorless and slightly viscous 4-bromophenethylamine liquid), with a yield of 30%;
[0061] 2) Synthesis of Intermediate 2:
[0062] Take 0.12mol of phthalic anhydride and 0.1mol of intermediate 1, mix and dissolve in 50mL of ethanol, reflux at 100°C for 12 hours, suction filter while it is hot, cool the filtrate to room temperature, and precipitate colorless crystals, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com