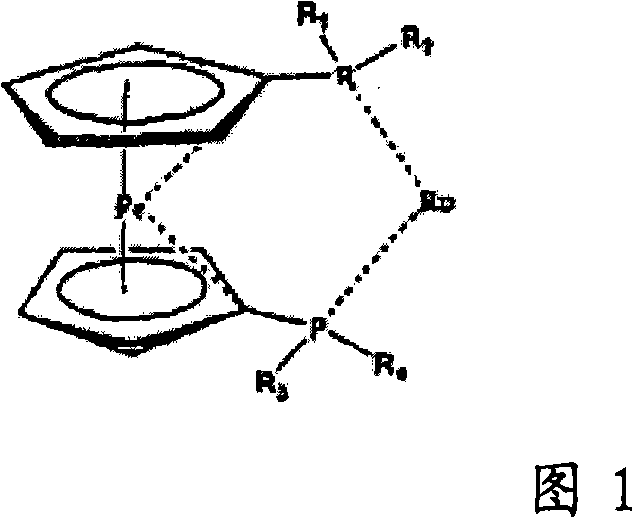
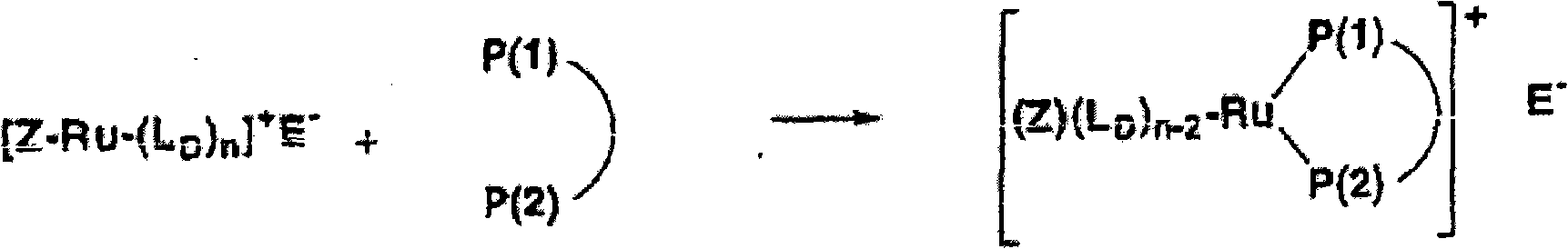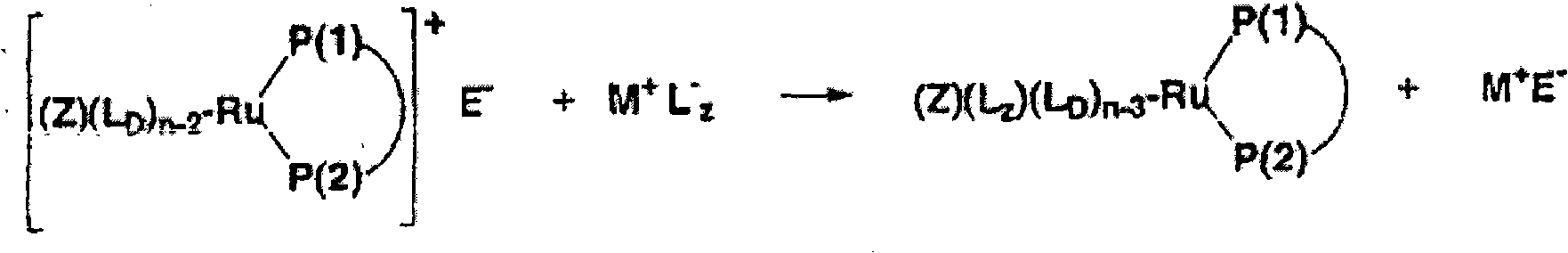Ruthenium complexes having (p-p)-coordinated disphosphorus donor ligands and processes for preparing them
A technology of ruthenium complexes and ligands, applied in the field of ruthenium complexes for homogeneous catalysis, can solve the problems of high consumption of catalysts containing noble metals, long hydrogenation time, low enantioselectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] (η5-2,4-dimethylpentadienyl) (CH 3 CN)-(Josiphos-SL-J212-1) Preparation of Ru(II) Tetrafluoroborate
[0079] a) (η 4-2,4-dimethylpentadienyl-η 2-C, H) (η 5-2,4-dimethylpentadienyl) ruthenium tetrafluoroborate
[0080] In a 100 ml Schlenk tube equipped with a magnetic stirrer, 1.1 g (3.77 mmol) of bis(η5-2,4-dimethylpentadienyl)ruthenium (UMICORE, Hanau) was dissolved in 50 ml of diethyl ether. Add 0.51ml (3.77mmol) of 54% HBF dropwise within 10 minutes at room temperature 4 -Et 2 O solution (from Aldrich). After the addition is complete, the mixture is allowed to settle, and the HBF is added dropwise by further 4 -Et 2 O to check whether the precipitation is complete. The upper solvent was removed, and the solid was washed twice with diethyl ether. The pale yellow residue was dried under reduced pressure. Yield 1.43 g (100%).
[0081] b) Acetonitrile complex (η5-2,4-dimethylpentadienyl) (CH 3 EN) 3 Preparation of ruthenium (II) tetrafluoroborate (starting com...
Embodiment 2
[0090] Preparation of (η5-2,4-dimethylpentadienyl)(iodo)-(Josiphos-SL-J212-1)ruthenium(II)
[0091] In a 25ml round bottom flask equipped with a magnetic stirrer, the acetonitrile complex (η5-2,4-dimethylpentadienyl) (SL-J212-1) ( CH 3 CN) Ruthenium(II) tetrafluoroborate (150 mg, 0.18 mmol) was dissolved in 4 ml acetone and 2 ml water and stirred with lithium iodide (LiI, 28.5 mg, 0.21 mmol) at room temperature for 3 hours. The solution was evaporated to dryness at room temperature under reduced pressure. The residue was washed three times with 1 ml of water. This gives the product in the form of a diastereomerically pure compound. Yield: 90%. pass 31 P-NMR spectroscopy verifies the P-P coordination of the SL-J-212-1.
[0092] Characterization:
[0093] 31 P NMR (CD 2 Cl 2 )δ: 7.51ppm (d, J PP =35.9Hz), 84.30ppm (d, J PP = 35.9Hz).
[0094] Use for catalytic hydrogenation
[0095] The (Josiphos-SL-J212-1)(iodo)ruthenium(II) complex prepared as described in Exampl...
Embodiment 3
[0097] (η5-2,4-dimethylpentadienyl) (CH 3 Preparation of CN)-((R,R)-DepyPhos) Ru(II) Tetrafluoroborate
[0098] In a 50 ml Schlenk test tube equipped with a magnetic stirrer, the acetonitrile complex (η5-2,4-dimethylpentadienyl) (CH 3 EN) 3 Ruthenium (II) tetrafluoroborate was dissolved in 15 ml of dichloromethane and stirred with 25 mg (0.05 mmol) of (R,R) DepyPhos (from Digital Speciallty Chemicals, Toronto, Canada) for half an hour at room temperature. Then 50 ml of n-hexane was added dropwise to the solution. A yellow-orange solid precipitated which was filtered off. Residual solvent was removed at room temperature under reduced pressure. The product was obtained in the form of diastereomerically pure compound, yield: 98%. pass 31 P NMR spectroscopy confirmed the P-P coordination of the ligand (thus the presence of a 5-membered ring).
[0099] Use for catalytic hydrogenation
[0100] The complex prepared as described in Example 3 was used for the asymmetric hydroge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com