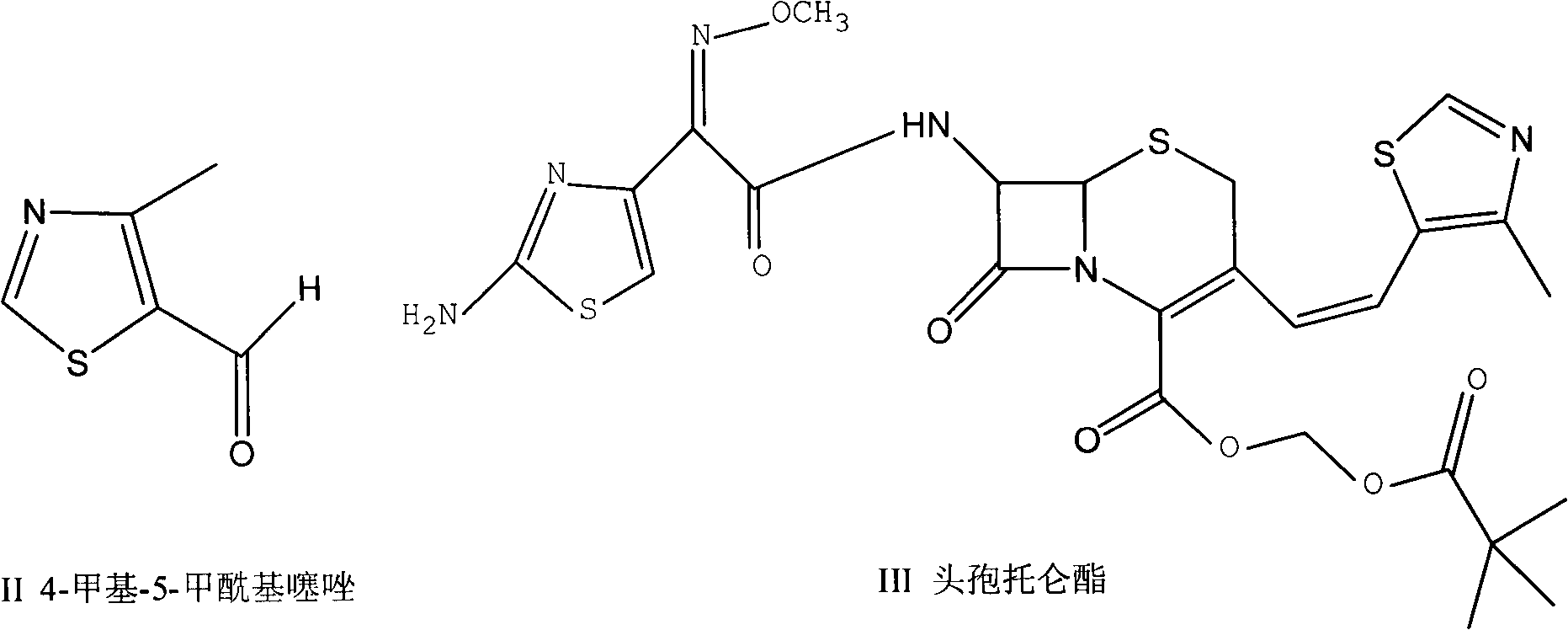
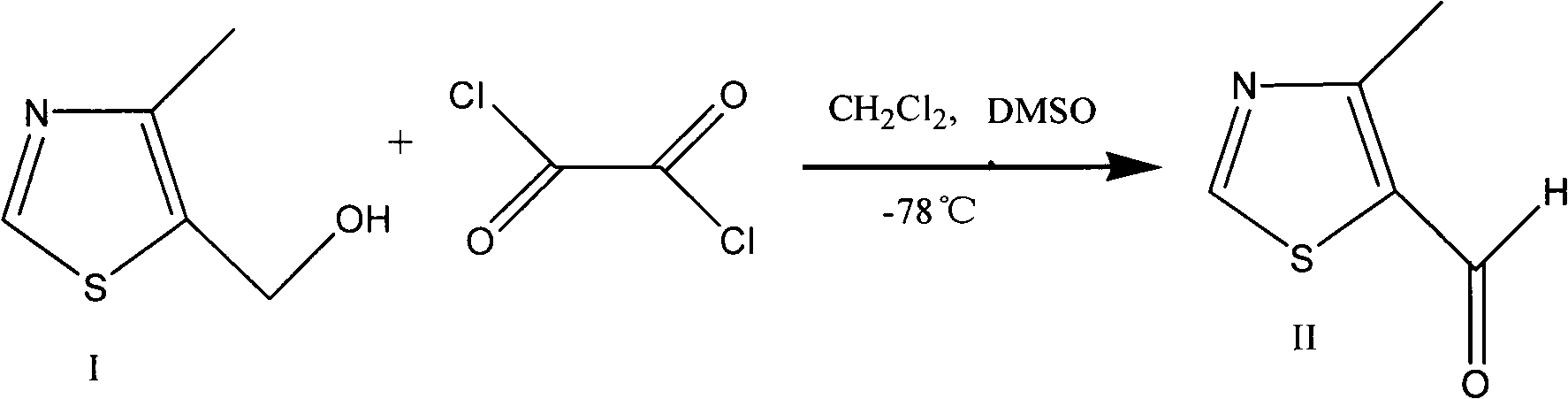
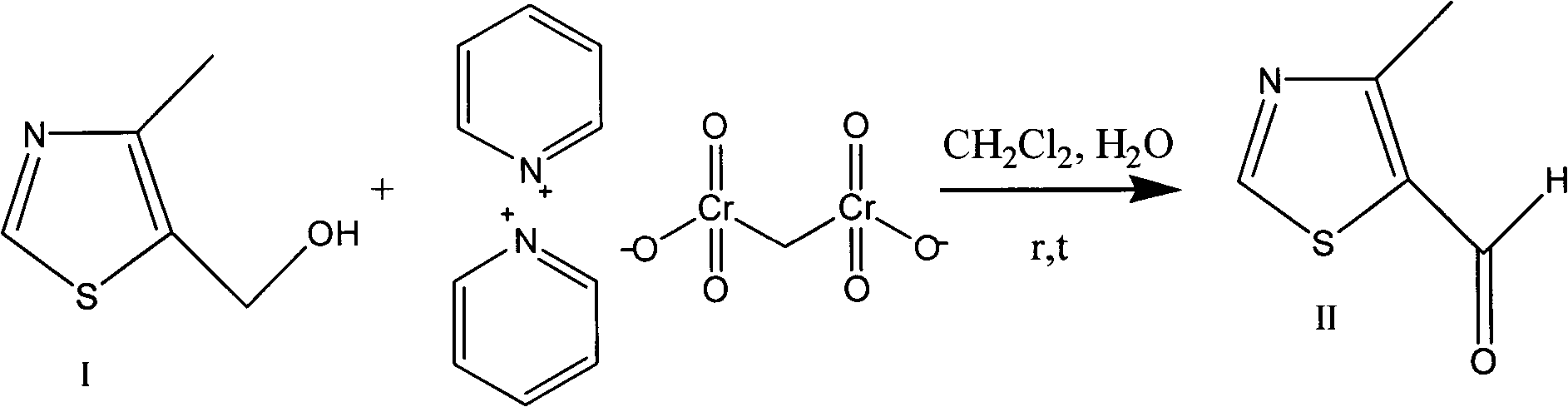
Preparation method of 4-alkyl-5-formoxyl thiazole or 5-formoxyl thiazole
A technology of formylthiazole and alkyl, which is applied in the field of drug synthesis, can solve problems such as unfavorable industrial production and environmental protection, raw materials containing heavy metal pollutants, harsh reaction conditions, etc., and is beneficial to industrial production and environmental protection, and has a wide range of applications , easy post-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 prepares 4-methyl-5-formylthiazole
[0034] In a 100 mL three-necked flask, 0.5 g (3.876 mmol) of the compound of formula I was dissolved in 17 mL of dichloromethane and 2 mL of tetrahydrofuran, and stirred at a temperature of 20°C.
[0035] A solution (pH = 8-8.5) prepared by dissolving 1.5 g of sodium bicarbonate in 15 mL of water was added to the above organic solvent.
[0036] While the biphasic solution was maintained at 20°C-25°C with vigorous stirring, 0.06g (0.384mmol) of 2,2,6,6-tetramethylpiperidine-1-oxyl radical (Tempo) and 2.2g (15.38mmol) of calcium hypochlorite, while monitoring the progress of the oxidation reaction by TLC.
[0037] After the reaction is complete, filter the biphasic solution, place to separate the two phases, wash the organic phase with 10 mL of aqueous potassium bisulfate (1.0 g of solution 10 mL of water), then wash with 10 mL of water, dry over anhydrous magnesium sulfate and reduce pressure The solvent was distilled o...
Embodiment 2
[0041] Embodiment 2 prepares 4-methyl-5-formylthiazole
[0042] In a 250 mL three-necked flask, 2.0 g (15.5 mmol) of the compound of formula I was dissolved in 85 mL of dichloromethane and 10 mL of tetrahydrofuran, and stirred at a temperature of 20°C.
[0043] A solution (pH = 8-8.5) prepared by dissolving 14.2 g of sodium bicarbonate in 142 mL of water was added to the above organic solvent.
[0044] While the biphasic solution was maintained at 20°C-25°C with vigorous stirring, 0.145g (0.928mmol) of 2,2,6,6-tetramethylpiperidine-1-oxyl radical (Tempo) and 6.6g (46.15mmol) of calcium hypochlorite, while monitoring the oxidation reaction progress by TLC.
[0045] After the reaction was completed, filter the biphasic solution, place to separate the two phases, wherein the pH value of the aqueous phase was 7.2 to 7.5, and the organic phase was extracted twice with dichloromethane (20mL × 2), the organic phase was combined, and the organic phase was Wash with 30 mL of saturate...
Embodiment 3
[0046] Embodiment 3 prepares 4-methyl-5-formylthiazole
[0047] In a 250 mL three-necked flask, 1.0 g (7.75 mmol) of the compound of formula I was dissolved in 43 mL of dichloromethane and 5 mL of tetrahydrofuran, and stirred at a temperature of 20°C.
[0048] A solution (pH = 8-8.5) prepared by dissolving 7.0 g of sodium bicarbonate in 70 mL of water was added to the above organic solvent.
[0049] When the biphasic solution was maintained at 20°C-25°C with vigorous stirring, 0.070 g (0.376 mmol) of 4-methoxy-2,2,6,6-tetramethylpiperidine-1-oxyl radical was added and 3.3g (23.08mmol) of calcium hypochlorite while monitoring the progress of the oxidation reaction by TLC.
[0050] After the reaction was completed, filter the biphasic solution and place to separate the two phases, wherein the pH value of the aqueous phase was 7.2 to 7.5, and the organic phase was extracted twice with dichloromethane (15mL × 2), the organic phase was combined, and the organic phase was Wash wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com