Process for refining group C/Y/W135 meningococcal polysaccharides
A meningococcal and polysaccharide technology, which is applied to antibacterial drugs, medical preparations containing active ingredients, and antibody medical ingredients, etc. No phenol residues, the effect of reducing endotoxin content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
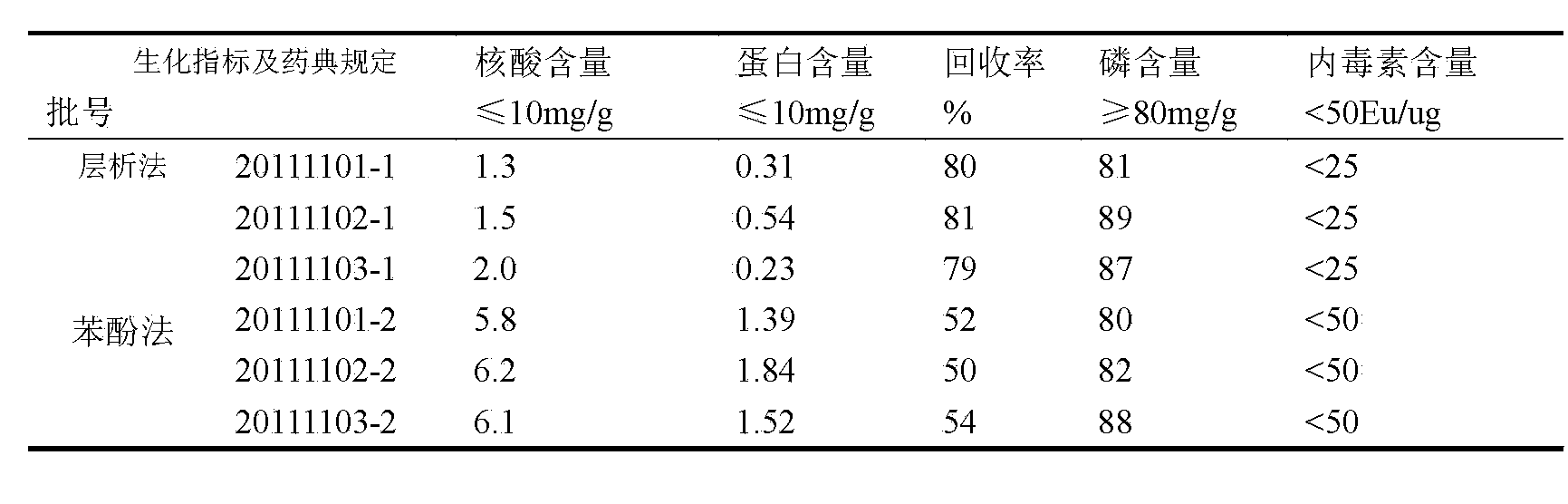
[0028] Example 1 Purification of group C meningococcal polysaccharide
[0029] The raw material is crude group C meningococcal polysaccharide, the solution is 10mM Tris-HCl, pH9.0; the equilibration buffer is 20mM Tris-HCl, pH8.0, which contains 1% DOC; the eluent is 20mM Tris-HCl , which contains 1M NaCl, pH8.0.
[0030] 1. Dissolve the crude group C meningococcal polysaccharide with a dissolving solution to 5 mg / ml;
[0031] 2. Use a SepHarose-G25 (26 / 10 desalting) column to replace the dissolved polysaccharide into the equilibrium buffer, collect the first peak of the external water as the polysaccharide peak, and the final concentration is about 2.5mg / ml;
[0032] 3. Put the collected polysaccharide solution on DEAE-4FF and Capto TM Adhere tandem column, the loading volume is 20~50mg / ml gel;
[0033] 4. Using linear elution method from DEAE-4FF and Capto TM The adsorbed substance was eluted on the adhere series column, the eluent used was 20mM Tris-HCl, pH8.0, and th...
Embodiment 2
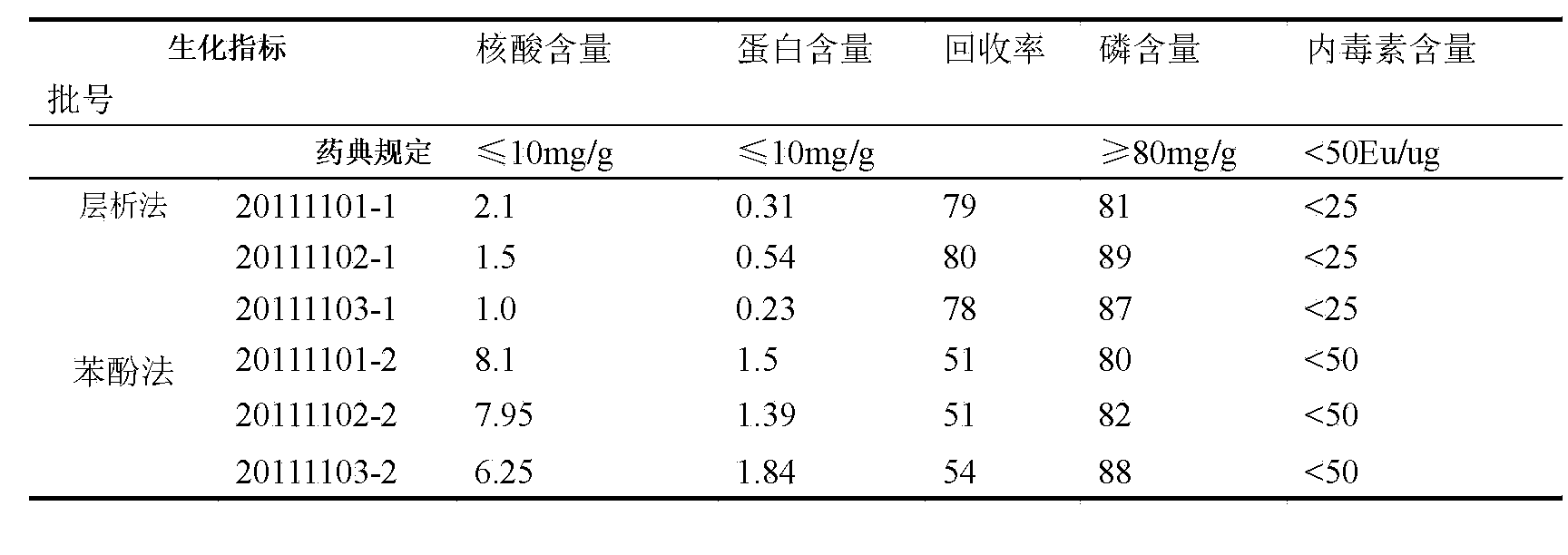
[0037] Example 2 Purification of group C meningococcal polysaccharide
[0038] The method as described in Example 1, the difference is that the aforementioned step 2 is changed to: use a 100KD ultrafiltration membrane, and replace the dissolved polysaccharide into the equilibrium buffer on the ultrafilter, with a final concentration of about 2.5 mg / ml. Compared with Example 1, this method can also achieve the effect of replacing the polysaccharide into the equilibrium buffer, and at the same time can omit the operation of collecting the elution peak, so it is more suitable for industrial production.
Embodiment 3
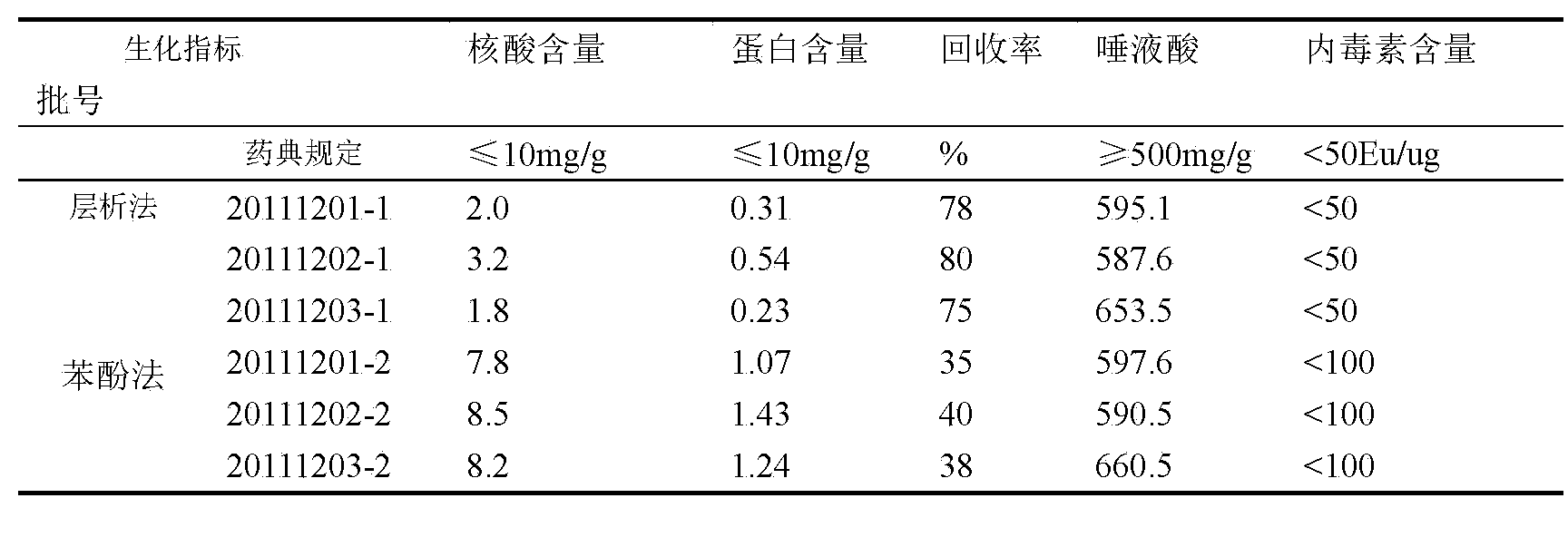
[0039] Example 3 Refinement of Group Y meningococcal polysaccharide
[0040] The raw material is crude group Y meningococcal polysaccharide. The solution is 10mM Tris-HCl, pH9.0; the equilibration buffer is 10mM Tris-HCl, pH8.0; the eluent is 20mM Tris-HCl, 1 mol / lNaCl, pH8.0.
[0041] 1. Dissolve the crude group Y meningococcal polysaccharide with a dissolving solution to 5 mg / ml;
[0042] 2. Use SepHarose-G25 (26 / 10desalting) column to replace the polysaccharide into the equilibrium buffer, collect the first peak of the external water as the polysaccharide peak, and the final concentration is 2.5mg / ml;
[0043] 3. Put the collected polysaccharide solution on Capto TM Adhere anion exchange column, the loading amount is 20~50mg / ml gel;
[0044] 4. Elute the polysaccharide by combining gradient elution and linear elution, specifically, gradient elution with 35% B eluent for 2 column volumes, and then linear elution with 35%-100% B eluent 5 column volumes. B eluent is 20mM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com